Leaderboard
Popular Content
Showing content with the highest reputation on 04/05/21 in all areas
-
2 points
-
2 points
-
As a general rule B. subtilis does chemotaxis in a different (sometime opposite) way versus E. coli. I never thought about bushings before, but I will ask around.1 point
-
OK so here is some more Chemistry which I hope will also be useful to Oneworld. As I have mentioned before, homegeny depends upon the scale you are working at. So starting inside the atom things are definitely not homogenous. There is a massive nucleus surrounded by a lot of empty space containing some electrons. Uniformity is represented by saying that every atom of a particular type is the same as every other atom of that type. For example all hydrogen atoms are the same. (for those who know about isotopes I am ignoring them) If we use those atoms to build molecules then again individual molecules are not homegenous since they are often made of different types of atoms. Even those that are combinations of atoms of the same type are not internally homogenous, in much the same way as atoms. So one hydrogen molecule looks much like another. We represent molecules by the 'ball and stick' models shown in exchemist's post, where the balls are individual atoms and the sticks represent the forces that hold them together. And yes, these forces are entirely electrical in origin. For small molecules again all molecules are essentially the same as with atoms and of definite composition, for example methane, ethane propane etc. Individual molecules can be isolated and, in principle at least, you could hand me one molecule of methane. So a large aggregate (number) of them is homogenous in that there would be the same number per cubic centimetre for each cc on your grid. Note that we live in a 3D world so we really need to work in terms of volume not area. This is why I talk of 'bulk clay'. However clays are in a different category. That of super large molecules. Super large molecules happen when a non specific number of atoms join together, unlike methane which always has 4 hydrogens joined to one carbon. A diamond crystal is an example of this. You could not, even in principle, hand me one 'molecule' of diamond. Another example would be a common salt crystal. Clays are like this only their structure is vastly more complex. But they all still can be represented by balls and sticks. As regards the sticks or (chemical) bonds there are several types available. The strength of these bonds are usually measured not in force units but in the energy needed to break them. Here is a table Now my table lists intramolecular bonds, which hold the atoms together in simple molecules and intermolecular bonds which can bind simple molecules such as water to each other hydrogen bonds. And yes the internal bonds in a single simple molecule are stronger than a single hydrogen bond, in general. But in large molecules there can be many many hydrogen bonds holding the parts together. For example in DNA the two strands or chains are held together by literally hundreds of hydrogen bonds this is called 'cross linking' Clays come in sheets rather than thin strands so the effect is even more marked. The subject is of such importance that books such as these have been written just about these clay minerals. Now to return to my original contention. For pottery the scale Chemistry works at is too fine and we should step up into the realms of mechanical properties. Here the clay may be considered homogenous for Pottery purposes since it will have come from a relatively thin piece of clay. I say this because geolically clay may have been laid down in very thick beds, up to thousands of metres thick. When this happns the clay may be stratified because as the particles which form it settle out of the water the larger, heavier ones settle first so will be at the bottom of any strata which mark the start and finish of a period of formation. But pottery type clays will be dug out within a couple of metre depths so will be homogenous. Next post we can examine the mechanical properties that demonstrate this question of homogeny and uniformity. You may have heard of the Atterberg Limits for field testing clays.1 point
-
Why would any science forum do that? You obviously miss the whole meaning of why science forums [most of them] exist. It's to discuss mainstream science obviously under the banner of the scientific methodology. That to many folk, [who lack the knowledge and vision as to how awesome and interesting science is] is geeky and boring. They are of course obviously wrong. What can be more exciting, awesome and invigorating, then discussing and learning about predictions made by GR 100 years ago, that have finally in recent times been verified. The problem [as I see it] is with some who come up with what seems like brilliant ideas and theories [to themselves], without actually knowing the data that already exists on that particular area of science, and then get all uppity and upset when their ideas and hypothesis are shown to fail under scrutiny. Many of those people with those ideas, also have some agenda to push. All new ideas and hypotheticals need to run the gauntlet, just as current accepted scientific theories have previously run the gauntlet. In that way forums such as this can be generally assured of "quality over dubious quantity"...isn't that what science forums such as this should be striving for?1 point
-
Thanks for bold underlining your goalpost move, but at a certain point, you need to acknowledge that any step in the right direction is a good step.1 point
-
Seems like a trend these days for some lower information types to assert their more bizarre ideas rather than sound them out and try to support them. You may not see this the way I have, but our members are ALWAYS willing to discuss almost anything if it's done in good faith. The trend is rampant in US politics as well. The new conservatives aren't content with compromise. All or nothing has become the norm. If they can't have their way, they want to knock the board over because the game must be rigged. Discussion isn't possible when one side has no means of being persuaded. In science discussion, evidential support is what makes us nod and say, "Go on...." If a new member can't provide any, are we supposed to keep talking about their idea? If we point out their mistakes based on our best current explanations and they ignore us, is there anything to learn? The point is discussing science. It's often plodding and boring to those who don't collect knowledge. I'm not going to apologize for it. We've lost some folks this last year, hopefully not to COVID-19, but because the virus made their expertise suddenly indispensable. We wish those folks the very best. We hope they can come back online and tell us about their experiences. Do well, as in more traffic? We could allow more crap, and that would attract more crappers. Do well, ala PhysicsForums membership size? They're even tougher on newbies, are huge because of advertisements, and don't allow speculation at all. Do well, as in be more rigorous and strive for more accuracy? We choose this. If we could gain another couple hundred members who're serious about mainstream science, this is what we want. We'll keep striving on a volunteer basis.1 point
-
1 point
-
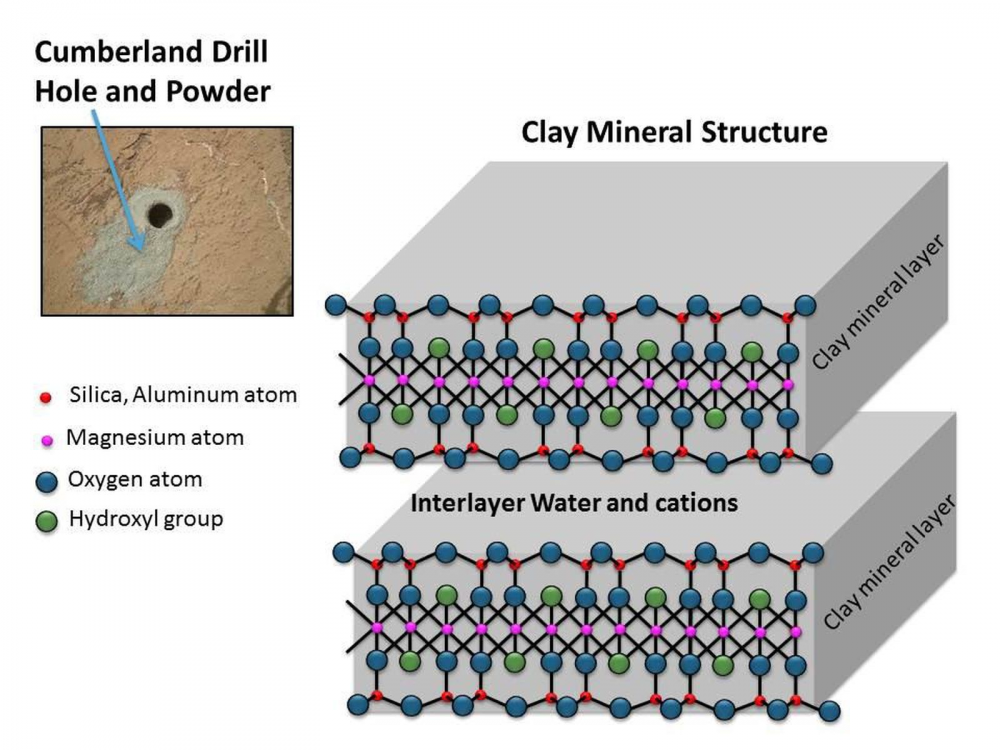
I'm not a mineralogist, but I can try to add a bit to what others have said, based on what I have quickly been able to read up.😉 Clays are made up of tiny crystals of "clay minerals" and water. The water is hydrogen-bonded to the surface of the crystals, which means it is attached by bonds that are about a tenth the strength of a full "normal" chemical bond. A crystal of dry clay mineral will tend to absorb water until there is a hydrogen bonded film of water all along its surface. Clay minerals are made of sandwiches of sheets of silicate tetrahedra, which have a -ve charge, with metal ions in between that have a +ve charge, thus making the whole sandwich electrically neutral, and then water molecules in between one sandwich and the next. (Though some are Danish open sandwiches with only one layer of silicate "bread" under the metal ions and nothing on the top.) Because they have a sheet structure, these minerals easily cleave along the lines of the sheets, as mica does. What you end up with is a lot of microscopic, flat, very thin flakes of clay mineral, with water in between. It is the water that makes clay plastic, enabling you to mould it, as it allows the flat flakes to slide past one another. In terms of chemical bonding, you have covalent bonding within and between the silicate tetrahedra, ionic bonding between the sheets of tetrahedra and the metal ions in the sandwich, and finally hydrogen bonding of water molecules along the outside of the sheets. So clays are chemically quite complicated things, dull though they may look from the outside. So when you ask if clay is uniform or homogenous, it depends on at what level you mean. Macroscopically it is, but at the molecular level it is made of two distinct phases, a solid mineral and liquid water - albeit much of the water is hydrogen bonded to the mineral so that it does not behave entirely like a liquid. Moulding the clay does nothing to this structure. However, drying or firing the clay will drive off most of the water, shrinking the clay and hardening it by allowing the layers to link together directly instead of being kept apart by a layer of water. Here's a diagram I found which may help visualise it:1 point
-
1 point
-
Why wouldn't you answer the question and instead say this?! I gave arguments and linked sources, all you did - was to strawman me. Why even answer, if you gonna do this? I don't know what debate tactics you are using! But simply repeating this: "The evidence rather strongly implies otherwise." Ad nauseam doesn't seem to me like pinnacle of a discussion on scientific forums, nor proves anything! If your believes about science would be true, you would want to refute my arguments, instead you are using logical fallacies... I guess because I don't have reputation here allows you to just say 1 word and pretend like everything I said is wrong... Cancel culture much??? I guess you are on level 1 of lower society: https://en.wikipedia.org/wiki/Positive_disintegration De Ropp {https://en.wikipedia.org/wiki/Robert_S._de_Ropp) about science game: Source: https://archive.org/stream/selfrealization/Robert-S-De-Ropp--The-Master-Game_djvu.txt He says essentially it is a pointless game and it is better to go entrepreneurial! And this is only from 20st. Now it is even 1000 times worse. We already live in post-apocalyptic era, democracy is dead! Globalism rules! Capitalism is ruining professionalism: https://bigthink.com/politics-current-affairs/capitalism-safeguarding?rebelltitem=2#rebelltitem2 Don't get me even started on neolibs... https://www.jstor.org/stable/25746358?seq=1 Today in western societies everything (meant metaphorically - almost everything) aka from large part is about: - money - power - status - individualism - consumerism And this legislative passes, it will allow neolibs to create their own state with its own municipal goverment, sure their 1st priority will be to promote scientific ideals! https://www.marketwatch.com/story/in-nevada-desert-blockchains-llc-aims-to-be-its-own-municipal-government-01613252864 BTW how are your science ideals holding now during COVID pandemic? When every pharma hogging vaccines for themselves. And patenting. BTW top 25 pharmas make more than top 25 IT companies in USA!!! WHO is incompetent, Powerless! As our political leaders! Moonshot is non-profit organization researching COVID vaccines based on pro bono funding and bootstrap grants. They share their research freely with scientific community! It was carried out using FAH https://foldingathome.org/foldinghome. which is platform for shared computing, they achieved more FLOPS than top 5 supercomputers in the world together, yet with lower environmental costs!!! Woah! They asked NIH for funding and they were refused, apparently it was not even worth of their time! Again this could be for various reasons so don't quote me on this... I Am just mentioning this as interestingness. So again science is largely social as like 80% of all jobs! Scientists are still human beings!!! Provide any counter-arguments, or do not answer!-1 points