Leaderboard
Popular Content
Showing content with the highest reputation on 03/08/25 in all areas
-
I think red 3 was banned recently. In many areas I don't think that this is true as a whole, it depends on the specific article and the authors. Generally speaking, older studies have a better chance to be validated or invalidated. What you would do is to see which papers are citing the study and what their (updated) conclusions are. It is less of an issue in the field of toxicology (for the most part). But in other areas I have noticed that in newer papers folks only cite within the last 5 to maybe 10 years. These are often younger researchers and in more than one (or a dozen) occasions, I found that they kept re-inventing the wheel, because they were not aware of older findings where the same effect was already shown.1 point
-
Aren't we still using those same dyes and colorants? I found a current list from the FDA: https://www.fda.gov/food/food-additives-and-gras-ingredients-information-consumers/types-food-ingredients1 point
-
I think many Americans have known that something major is wrong for quite a while, but we've all been so indoctrinated NOT to blame the wealthy that it's pretty easy to distract us with various "enemies from within". Some saw the insurrection as just a repudiation of the status quo, and failed to sense the significant threat to the democracy (or they may blame democracy for not giving them more than a single vote). I've had to explain to MAGAts that the insurrectionists didn't just protest the vote count in accordance with their constitutional rights, they did something the Constitution absolutely forbids them from doing, and NONE of them already knew that. What's shocking is the pace the Republicans are setting for destroying the American culture and economy. I don't understand how the big corporations figure they'll prosper when nobody has any money left to buy their products, either from price gouging or losing their livelihood or having their pensions and Social Security robbed. These corporations don't make much off the 1%, it's the rest of us that keep them profitable, so where is this leading? Does anybody in corporate land think it's a good idea to remove spending power from your customer base? Or are they all sitting on so much cash that they feel they don't need us to consume any more?1 point
-
! Moderator Note We don't need a speculative approach to this conversation. There have been studies on this, so I'm moving this to Applied Chemistry. https://pubmed.ncbi.nlm.nih.gov/23026007/1 point
-
What was behind my question was the degree to which the average Trump voter really thought he or she was voting for authoritarian government and the suppression of the checks and balances of liberal democracy. My impression is that a lot of voters are pretty unengaged and just vote on the basis of things like gas (or egg?) prices. It seems shocking that they could vote for someone that tried to overthrow the result of the last election, but did they actually think about that?1 point
-
1 point
-
It’s hard to tell if this is a serious question. Partly because of your “jokes” and partly because you don’t seem to have done a lick of research on the question. Incessant whining, based on ignorance, is hard to take seriously. The Apollo missions cost more than $300 billion in today’s dollars. (2023) https://taxfoundation.org/blog/apollo-moon-space-race-industrial-policy-cost/ (original cost was ~$25 billion) This shows that each mission cost less than $500 million, so even if you shave off the last 6 missions, that’s less than 1/8 of the overall cost (3 billion out of 25) https://www.statista.com/statistics/1028322/total-cost-apollo-missions/ So the new program is a lot cheaper, because certain things don’t have to be re-discovered, even as the hardware is remade with modern technology. But it’s not simple and it’s not easy.1 point
-
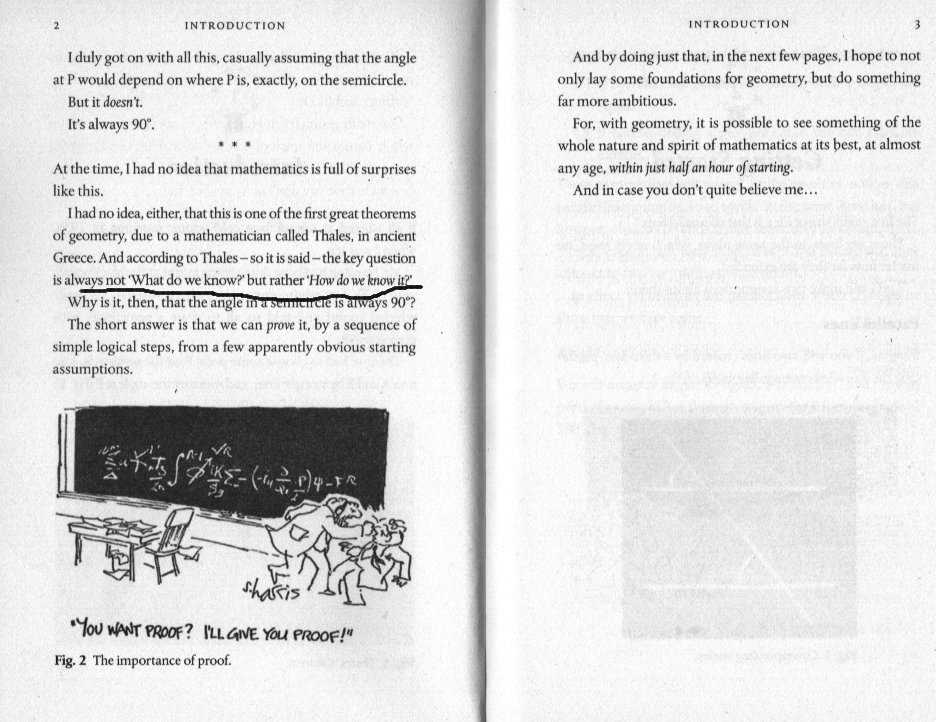
Please remember you are not the first to ask 'why ?' or 'How do we know that ?' It is a very reasonable question. Here is an introductory page from a newish book by a well respected maths author. You may be suprised to learn that Thales, a well respected Greek philosopher wondered exactly that several hundred BC. I have underlined the important sentence. Now that we have established that experimenters originally started by naming things in front of them and working from there perhaps we can revisit the posts I made about why Dalton thought of atoms and molecules and The law of constant proportions or definite proportions. You didn't say if you followed the reasoning in those posts. Once we have that out of the way we can move on to what is inside those atoms and molecules.1 point
-
He probably got it from a chemical supplier, and he knows it's silver nitrate probably because that's what it says on the label of the bottle it came in. My point is that modern day chemists do not start from scratch. They (to use a well-known quote) stand on the shoulders of giants. It should be noted that knowledge of chemistry developed over time and in parallel with knowledge of physics. I think that the scope of your questions is too large for anyone here to provide you with genuine help, as much as they may try. I recommend that you study a chemistry textbook aimed at school children. However, you may find this Wikipedia article interesting (though I haven't fully read it myself): https://en.wikipedia.org/wiki/History_of_chemistry1 point
-
OK, hydrazine. This is N₂H₄, https://en.wikipedia.org/wiki/Hydrazine so made of molecules each of which has 2 atoms of nitrogen, joined by a single chemical bond N-N and with each nitrogen atom also being to joined by a bond to 2 hydrogen atoms. The astronauts and rocket scientists know what it is because it is made for them in a chemical plant, but if your question is how can someone , in principle, test this stuff to confirm its identity, then I think one would probably have a look at its infra-red spectrum. Molecules like this absorb infra red radiation at particular frequencies, according to the atoms present and how they are bonded. (The bonds are stretchy, so if the atoms are pulled apart or pushed together, the bond can be set into vibration, the frequency of which depends on the mass at either end and the strength of the bond. Infra red waves of the exact frequency required can pull or push them in this way and when they do the molecule absorbs some of the radiation, which the spectrometer can detect.) But your more general question is really about analytical chemistry as a whole. This is a big subject. The various forms of spectroscopy, one of which I have described above, play a big role in helping to identify chemical compounds. But there are also other methods which often involve trying to carry out chemical reactions to see what results. Normally this only works when you already have some idea of what you are looking for. I think your last question, about Mg and the significance of the number 12, actually gets us to an excellent starting point for some understanding of chemistry, because it gets us to the Periodic Table of the elements. Here's a link to one I use for reference: https://ptable.com/#Properties Mg is the chemical symbol for magnesium and it is the 12th element in the table. 12 is its "atomic number". And you are right, the atom has 12 electrons, to balance the 12 positively charged protons in its nucleus. Chemistry is all about the electrons in the atom: electrons are what form chemical bonds. The number of electrons in atoms of the various elements determines how each element will behave, chemically. Mendele'ev, who originally designed the Table in the c.19th, did so without knowing this (!). He just observed there were similarities in the chemical behaviour of certain elements and grouped those into columns. So for example, the column at far left with lithium, sodium etc. are all very reactive, soft metals which react with, say chlorine to form white salts with one atom of chlorine per atom of metal. (Common salt NaCl is one example.) The next group, with Mg, Ca etc in it, also form white salts but with two atoms of chlorine per metal atom. So he realised there is something important in common between Li and Na, and between Mg and Ca. The rest of the table was built up in similar fashion, from knowledge of the reactions of elements and the compounds they tended to form. Nowadays we know it is to do with the way electrons build up in layers ("shells") like an onion, as one moves from lighter to successively heavier atoms, so that repeating patterns come back over and over when the shells are similar. This is something we can discuss. Elements with higher atomic number have more mass, so as one reads the table starting at the top left, one gets a progression of successively heavier atoms. The table is divided nowadays into blocks, according to the types of properties one finds in the columns (known as periodic table Groups). Again, this is something we can discuss. If you click on an element in the version of the table I have linked, you will get on the left a rather technical summary of key data but also, probably more interesting to you, a blue link to a Wiki text article all about the element.I suggest having a look at magnesium, since you asked about it, and perhaps also nitrogen, since you asked about hydrazine.-1 points
-
By 1875 they had a pretty good idea of how to characterise chemical compounds. Mendele'ev's periodic table came out in 1871. The basic way the c.19th chemists worked was by careful measurements of weight changes. They weighed the reactants and the products and from this were able, eventually, to work out the atomic weight of each element. Once they knew the atomic weights, they could use weight changes during reactions to establish the chemical formula for each substance. It all started back in the time of Lavoiser, at the end of the c.18th (he lost his head in the French Revolution, in fact, poor fellow). He studied combustion, through which he was able to find out things like the fact that when you burned something it absorbed only 20% of the volume of air available. So that told him there were 2 components in air, the 80% component being inert. He called it "azote", from the Greek for lifeless, which is French for nitrogen to this day. He also found the ash from burning phosphorus weighed more than the phosphorus before burning, and that this ash was acid when dissolved in water. So he realised the component of air that reacted had become part of the ash, along with the phosphorus - a chemical compound. Because the ash was acid he called the reacting component of air "oxygene", from the Greek for acid-generating. What he had made was phosphorus pentoxide P₂O₅, and when he dissolved it, he got a solution of phosphoric acid. (Lavoiser was subsequently shown to be wrong in associating oxygen with acidity, when hydrochloric acid was shown not to have any oxygen in it. In modern chemistry, acids are substances that release hydrogen ions, H⁺, in solution. So it was 2 steps forward, one step back.) It was by painstaking experiments like this, carefully weighting reactants and products, or carefully measuring volume changes in the case of gases, that by degrees the identities of chemical elements and compounds and their formulae came to be discovered, from the proportions in which they reacted.-1 points
-
I like these questions. When one is familiar with an area of science it is easy to ignore the original basis of the concepts one uses all the time. Some of these questions force me to go back and review the history of it all, which is informative. 🙂 OK, a reactant is something that takes part in a chemical reaction. For instance if you burn hydrogen, it reacts with oxygen and produces water: 2H₂ + O₂ -> 2H₂O. Hydrogen and oxygen molecules, shown on the left hand side of the chemical equation are the reactants and water, shown on the right, is the product of the reaction. So one quite commonly speaks of reactants and products. Yes good balances were available, as they were needed by gold and silversmiths for assay purposes. Joseph Black seems to have been the first to use one for chemical purposes at the end of the c.18th. He had one that was accurate to 0.1g, apparently. So by the mid c.19th I expect they had them good for 0.01g or so, which is fine for the sort of thing they were doing. Re phosphoric acid, Lavoisier would obviously have known he had made an acid derived from phosphorus. But the modern term phosphoric acid probably would not have been applied to it until later. Regarding atomic weight, you will see that shown for each element on the periodic table, along with atomic number. It works like this: Atomic number denotes the number of protons in the nucleus. In a neutral atom this is the same as the number of electrons. When it comes to atomic weight, electrons are so light their mass is negligible, at least in chemistry. What counts for atomic weight is the numbers of protons and neutrons in the atomic nucleus. Protons and neutrons weigh almost the same and the number of neutrons in a typical nucleus is normally about the same as the number of protons, though with some variation. So the atomic weight is generally about double the atomic number. Atomic weight is quoted in units of protons or neutrons. For example the atomic number of oxygen is 8. The atomic weight, on the table I linked to in my earlier post, is given as 15.999, so almost 16 but not quite. The reason why it is not exactly 16 is because although almost all oxygen atoms have 8 neutrons, a very small proportion have a different number. The number shown in the table is an average. (These nuclei with different numbers of neutrons are different "isotopes" of oxygen, from the Greek for "same place", i.e. they have different masses but occupy the same place in the periodic table. That is because, so long they all have 8 protons, and therefore 8 electrons, they have identical chemical behaviour - which is what defines them as the element "oxygen". ) This post is getting long, so I'll start a new one about ions. Right, ions. An ion is an atom in which the number of electrons is not the same as the number of protons in the nucleus. That means it will have a net electrical charge, +ve if it has fewer and -ve if it has more electrons than needed for electrical neutrality. I gave an example earlier with H⁺. This is a hydrogen atom with its one electron taken away, leaving just a proton. (If you've ever had an ulcer, you may have been prescribed a "proton pump inhibitor". This just means something that inhibits the mechanism in the stomach that secretes acid, so fewer protons, or hydrogen ions (H⁺) are produced.) You can have ionic compounds: common salt is one. It consists of equal numbers of +ve sodium ions and -ve chloride ions, which attract one another and form a regular pattern in a crystal of salt. Both of these have lost or gained one electron per atom, but higher numbers are possible, depending on the element , e.g. Ca²⁺ or O²⁻ . One way you know you have got ions is if you dissolve the substance in water and see if it conducts electricity. Salt water conducts well, because +ve sodium (Na⁺) ions are attracted to the -ve wire and chloride (Cl⁻) ions to the +ve one, so a current can flow. (By the way the chemical symbol for sodium, slightly annoyingly, is Na, from the Latin natrium. The word originally comes from Wadi El Natrun in Egypt, where natural deposits of sodium carbonate are to be found. As Michael Caine would say, "Not many people know that".)-1 points
-
I’m going to break up the replies so we don’t get a lot of hares running in different directions. First, Lavoisier didn’t have anything like spectrometer, back in the 1780s. He would have recognised phosphorus from its physical and chemical properties, as was done for all chemical substances before spectroscopy became an analytical tool, which was in the 1st half of the c.20th. I’ll come back to the discovery of protons and neutrons later. But as to the question of weight of atoms, that comes down to weighing a sample of a substance and working out how many atoms are present in the sample. For that we need to introduce the concept of Avogadro’s Number, which is extremely important in chemistry and deserves its own post. So I’ll come back to that a bit later today, too.-1 points
-
OK, regarding weighing atoms - and thereby the protons and neutrons that make them up - this comes down to knowing what number of atoms there are in a given weight of substance. Avogadro's number is the standard number used in chemistry. It defines what is called a "mole" of the substance. ("Mole" comes from German "Mol", which was a term derived from "molecule"). One mole of any substance contains 6.02 x 10²³ molecules of it (or atoms of it if it is an element). This extremely large number is Avogadro's Number. The number is chosen such that the weight in grammes of one mole is equal to the atomic (or molecular) weight of the substance. For example, one mole of carbon weighs 12g and contains 6.02 x 10²³ carbon atoms. (If you look it up you will see the atomic weight of carbon is 12.) One mole of water contains 6.02 x 10²³ molecules and weighs (2 x 1 + 16) = 18g, because its formula is H₂O, and the atomic weight of H is 1 and that of O is 16. It is very important to know the number of moles of a substance when considering chemical reactions, because that determines the proportions that will react together. For instance, taking water again, the formula H₂O means that 2 atoms of hydrogen are combined with one of oxygen in each water molecule. So in, say, the combustion of hydrogen, which produces water, 2 moles of hydrogen atoms will require one mole of oxygen atoms. In terms of weight, every 2g of hydrogen will take up 16g of oxygen. So when you do a lab reaction, you can weigh out the proportions you need and not have unreacted material left over at the end. Coming back to protons and neutrons, In practice, nobody tries to express the mass of a proton or a neutron in grammes. But from the above I hope you can see we do have the relationship between the number of protons and neutrons in an atom and the weight of a set number of them in grammes, via the mole and Avogadro's Number. There is a nice little write-up here of how Avogadro's Number came to be determined: https://www.scientificamerican.com/article/how-was-avogadros-number/ Admittedly it refers to a number of other pieces of physics and chemistry, but that's the way with science: everything is interlinked and feeds off other stuff. So to learn it there are times when you have to decide not to follow up all the loose ends at once, or you risk getting lost in a swamp of information. I'll come back to the question of ions separately, so that we can keep these strands of Q&A apart. Actually this is doubtful. A flame test requires a steady, non-luminous flame. This became available for the first time in the mid c.19th with the Bunsen burner.-1 points
-
Take a look at the periodic table link I gave you and read up the physical and chemical properties of phosphorus. It's a bit complicated because there are different "allotropes" of phosphorus (from the Greek for other forms), but you can get an idea of what its characteristic features would have been, for someone of Lavoisier's time. I don't know which form of phosphorus he was dealing with, I'm afraid. But these guys were not mugs. Elements like sulphur or mercury, were known to them, from all the experiments the old alchemists used to do, in the course of their fruitless quest to turn lead into gold.-1 points
-
It's good for quite a few metals but doesn't help for everything. And there's also the problem of colours that look similar. But it is a useful thing. As @studiot says, it can be thought of as a very basic kind of spectroscopy. On the mole/Avogadro's number stuff, yes it is a lot to take in but we've covered quite a lot of ground already, thanks to your questions. Let's review it: - the periodic table and why it is structured the way it is, with similar properties among elements in each of the groups (columns) - protons, neutrons and electrons and what makes a element an element - Atomic number and atomic weight, and isotopes - atoms versus ions. - Avogadro's Number and moles, and their importance for the ratios in which substances react together. - Plus some history of the start of chemistry with Lavoisier's studies of combustion That's quite a lot already. This would have been several weeks of school study for my son, for instance. I realise I have not come back to you yet on how we know ions are ions. There is probably a missing piece on the history of how the concept of atoms and molecules was validated, considering they are far too small to see directly (the wavelength of visible light is of the order of 500nm, whereas an atom is of the order of 0.5nm across, so 1000 times smaller.) Ions are just electrically charged atoms, so if you accept the existence of atoms and find that certain solutions conduct electricity, there must be charge carriers of some kind, so it's not a huge leap to think there are atoms with electric charge.The substance produced at the electrodes are also an indication of what is happening, e.g. chlorine is evolved at the +ve electrode when a current is passed through a salt solution. The Cl- ions give up their extra electron to the electrode and make chlorine gas.-1 points
-
All good info, save that this is not what a "functional group" is in chemistry. That term is used in organic chemistry, to denote groups of atoms which are part of an organic molecule, conferring specific types of behaviour, or functionality. Examples would be amine groups or carboxylate groups. Acid/base and redox classifications would not be described as functional groups. More here: https://en.wikipedia.org/wiki/Functional_group I don't understand your question about silver nitrate. This video does not relate to the dawn of chemistry in the c.18th, when there were real issues with correctly identifying the substances they worked with. For the last 100 years at least, people have been able to obtain chemical compounds from commercial suppliers. Are you serious or are you messing us about?-1 points
-
I’m only kidding. I am making fun of the fact DEI is extreme in the case of specifically putting a women on the moon just because it never happened before. I do see a need because I don’t believe women were as astronauts till 1983. I do not object to a woman on the moon. I just making fun of the fact it costs so much money. Actually I am very interested in space. I wouldn’t want to be an astronaut though. It would be like being in a submarine 6 months. It isn’t like Star Trek yet. And it is extremely dangerous. NASA is great for learning and exploring but they too have safety and expense issues. They launched the Challenger shuttle after it had 6 ft ice sickles on it. i don’t like the focus to be the first woman on the moon. She is part of the mission and the focus should be building on the moon. The current NASA is very diverse. And if you go to Kennedy Space Center and look at the Wall of Heroes, you will be impressed by how qualified they are. So I see why I made fun of the price tag to go to the moon. But in my lifetime NASA has always been diverse. The fact that it is so dangerous makes it hard. The lunar lander has just landed on its top and the SpaceX rocket 🚀 exploded. Have you ever heard of ideas for a space elevator? Could such an elevator climb so high then fall to the other side of the sphere?-1 points
-
Inflation exists bc we all insist that growth is essential and then we all make bets and the house wins, until it doesn't... 😉-1 points
-
I know, for example, that Russian government prints a lot of money for funding the Putin's war. It is really strange if you deny that the quantitave easing is the cause of inflation. Ok here are the words of Arnold Swarzenegger about the money printing: This was a link with timecode, does this forum supports the timecodes on youtube? If no, the start is at 26:06.-1 points
-
Well the video highlighted the mission would highlight that women and minorities lead the mission. Which is ok. I am just poking fun of the fact that it is costing a 100 billion dollars. This mission is way behind. If we were on the moon in 1970’s why is it so hard to go back? Yes I know these women are good astronauts. Putting someone on the moon is great, but if you say first woman on the moon, $100,000,000,000. Eliminating the need for DEI, priceless.-1 points