Everything posted by Sensei
-
Temporal Uniformity
How about radioactivity? [math]m(t)=\frac{m_0}{2^\frac{t}{t_{1/2}}}[/math] m0 - initial mass of radioactive isotope f.e.[math]^{14}_6C[/math] https://en.wikipedia.org/wiki/Carbon-14 t1/2 - half-life for [math]^{14}_6C[/math] is 5730 years. so after 5730 years we find that [math]m =\frac{1}{2}m_0[/math] There is no motion involved in this calc. We find animal body, carbon ore, ancient artifact, we know how much of Carbon-14 it should have, and measure what is actually mass of this isotope, and finding out how much of time was needed to decay isotope to have such effect. Reverse of above equation. Instead of measuring mass m at time t, measure time t from known m0 and m and t1/2.. Radioactive dating article: https://en.wikipedia.org/wiki/Radiometric_dating
-
Temporal Uniformity
One can move in +1m in X axis in f.e. 1 second, or 2 seconds, or 1 hour, or +1m Y axis in 1 second, or 2 seconds, or +1m Z axis in 1 second, or 2 seconds, (or any other distance d, less than c, in time less than t=d/c) Then how can you say "and thus no fourth dimension is needed to explain it.".. ?
-
Temporal Uniformity
Correction: you can't do it for bound-to-atom electrons. But you can do it for free electrons, traveling through vacuum, or through cloud chamber or other particle detector and leaving trace (and slowing down while traveling through medium). The more kinetic energy had particle, the longer trace. Long thin traces are from electrons. Short thick traces are from alpha particles (Helium-4 nucleus) But can you describe where is each atom in every molecule.. ? Either no. For polar molecule it's to some level of precision possible, because they have one region of molecule more positive and opposite more negative. So after flying though external electric field they rotate accordingly to electric field (other electrons or protons gathered on metal electrodes). But for perfect non-polar, it's pointless, as protons and electrons in molecule cancels each other nicely.
-
Temporal Uniformity
Mordred, you think your links are absolute truth, undeniable dogmas. There is needed more humble opinion. Don't reject experimental evidence prior you know it first.. (whatever it is).. f.e. Dark flow https://en.wikipedia.org/wiki/Dark_flow ps. You don't know, what you don't know..
-
Temporal Uniformity
It's way more complex than you may think. Say we have gamma photon with energy exceeding 1.022 MeV energy (f>=2fC), once it will collide with stationary atom (stationary in it's own FoR), there is created electron-positron pair. [math]\gamma \rightarrow e^+ + e^-[/math] But we have something like redshift and blueshift Relativistic Doppler effect. [math]f=f_0\sqrt{\frac{1+\frac{v}{c}}{1-\frac{v}{c}}}[/math], blueshift [math]f=f_0\sqrt{\frac{1-\frac{v}{c}}{1+\frac{v}{c}}}[/math], redshift So, if our atom prior collision with photon, is accelerated to significant speed of light, photon that had not enough energy, in "normal" circumstances, will be blueshifted, and in accelerated FoR will have enough energy for pair-production.. ps. It's all about definition of "what is mass", "what is energy", "what is length" and "what is time" (regardless of what swansont is saying it's not definable in numerous of threads). Unlike him, I think they're essential questions in quantum physics.
-
what's a good programming language to learn?
I thought so meaning of "official software" and "unofficial software" is obvious. Official version of software is made and released by original authors, or company which currently have copyrights for product. Unofficial version is made by some volunteers. And typically can't be sold, because it would violate copyrights of original authors. f.e. if I would take source of Apache, add something to it, compile, it would be my own unofficial version of Apache. Official Apache versions are released by Apache Software Foundation. No. Not generally. But Microsoft is author of .NET Framework and owner of copyrights, so in this particular case it's official. In the case of API, it has slightly deeper implications: original authors have original sources, and know everything in details about product. Unofficial are relying only on released API docs. So if something has not been mentioned in docs, unofficial version of f.e. language, will be incomplete, and might cause issues with certain software which utilized these not documented features.
-
what's a good programming language to learn?
.NET Framework is official product of Microsoft.. PHP authors compiled by themself their product to Linux, Microsoft did not.
-
what's a good programming language to learn?
You didn't understand. I was talking about running C# and .NET Framework on Linux as a 3rd party unofficial hack.. LAMP is fine. Runs natively as their original authors intended and compiled to Linux..
-
what's a good programming language to learn?
PHP is running on Linux natively, while C# doesn't have official support for Linux by Microsoft. Just some 3rd party hacks... Yet another thing to worry about whether particular code will work or not.. And yet another dependency on 3rd party.. And you have to have administrator privileges to install it in the first place. Web-servers are typically Unix/Linux machines.
-
The Official JOKES SECTION :)
Top Gear 4 Episode 2: Richard Hammond hypnotized and can't drive car: https://www.youtube.com/watch?v=B92M2Fy7zMk Here is what happened later in studio (start at 3 min): (unfortunately can't find any better quality on YT)
-
The Official JOKES SECTION :)
Jamie Oliver visits ordinary USA primary school...
-
What are you reading?
Dune is actually series of 6 books made by Frank Herbert (and even more by his son). http://en.wikipedia.org/wiki/Dune_%28franchise%29 Reading 1st volume is just beginning. It's hard IMHO (1st the easiest).
-
The Official JOKES SECTION :)
Gibraltar vs Ireland match: https://www.youtube.com/watch?v=s3i8h9N8Gr8 (I hope so YouTube won't delete it quickly)
-
Solar fusion, neutrinos and age of solar system
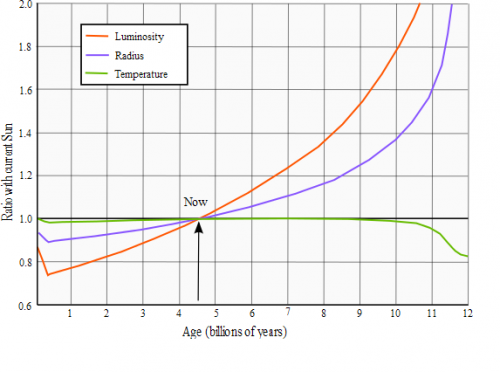
Using this graph we can calculate energy that Sun emitted in all its predicted by model lifetime. We can draw three figures. Triangle 1: [latex]A_1 = \frac{1}{2}*(0.9-0.75)*0.4 = \frac{1}{2}*0.15*0.4 = 0.03[/latex] Triangle 2: [latex]A_2 = \frac{1}{2}*(1.0-0.75)*(4.56-0.4) = \frac{1}{2}*0.25*4.16 = 0.52[/latex] and rectangle: [latex]A_3 = 0.75*4.56 = 3.42[/latex] (1.0 represents power of current Sun) Sum of their areas: [latex]A = A_1+A_2+A_3 = 0.03 + 0.52 + 3.42 = 3.97[/latex] [latex]\frac{3.97}{4.56} = 0.87061 = 87\%[/latex] of idealized energy emission from 1st post. [latex]P_{average} = 3.8651*10^{26} W * 87\% = 3.365*10^{26} W[/latex] Energy emitted for the last 4.56 bln years: [latex]3.365*10^{26} * 60*60*24*365.25 * 4.56*10^9 = 4.8423243744*10^{43} J[/latex] So, instead of [latex]9.17*10^{37}[/latex] Helium-4 atoms produced per second there will be average [latex]7.9835^{37}[/latex] [latex] t = \frac{1.101*10^{56}-8.1978*10^{55}}{7.9835^{37}}=3.52252*10^{17} seconds = 11.13[/latex] bln years
-
Soft "Science" and Evidence of Your Own Eyes.
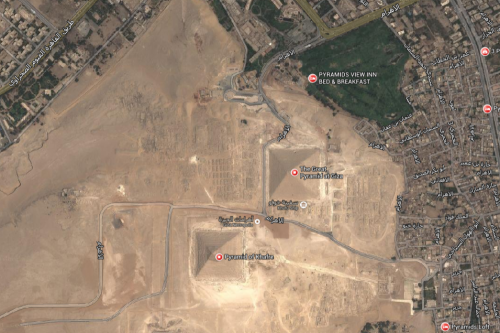
Remote desert? It's just 8 km in straight line from Nile.. Currently the first green trees are 100 meters from Pyramids.
-
Soft "Science" and Evidence of Your Own Eyes.
Imagine such device: Each block of rock used to build has average 2.5 tons. We can lift it up on little "boat", place rock on it, surround it by wood walls, fill container with water, and block is 1 level up. It's moved to it's place. Water is released. Boat goes down. New block of rock is placed on boat, and it's filled by water again, and cycle is repeated. We can imagine row of such water-lifters, around whole pyramid. Obviously when one level is finished, new lifters have to be build on above level. We would have to pump water to the top most lifter. Egyptians were using pumps since ever to irrigate grain fields, so technology is present. Water released from lifter in above level, can flow to below lifter, so it's reused, and less water have to be pumped.
-
Soft "Science" and Evidence of Your Own Eyes.
Have you bothered reading Rosetta Stone article, I provided? There are 3 languages used on it, and more or less, the same content.
-
Soft "Science" and Evidence of Your Own Eyes.
What a nonsense you are writing.. How we would know names of kings and pharaohs without being able to identify symbols.. ? Have you heard about Rosetta stone? http://en.wikipedia.org/wiki/Rosetta_Stone
-
Solar fusion, neutrinos and age of solar system
Neutrinos are taken into account. See below "Sum of energies". I am adding just once 0.42 MeV, assuming 50% of energy is taken by neutrino. 73.46% Hydrogen and 24.85% Helium from photosphere are pretty accurate measurements (similar content has Jupiter), as they're obtained from spectral lines of light from the Sun. What is inside is prediction from models, and will remain this way. After all we won't send there any device to check it experimentally.
-
Solar fusion, neutrinos and age of solar system
That's true it's unlikely to collide. But as I showed at the beginning of post, it must happen [latex]9.17*10^{37}[/latex] times per second anyway. Otherwise Earth would be able to get 1367 J of energy per meter^2 per second. Helium-4 fusion is even more unlikely to happen, because it requires 3 atoms at the same time (Be-8 has more mass-energy than 2 He-4 alone, and additionally decays to 2 He-4 + 92 keV, very rapidly): [latex]_2^4He + _2^4He + _2^4He \rightarrow _6^{12}C + \gamma + 7.275 MeV[/latex] More likely is fusion between He-3 and He-4 (if your suggestion about larger amount of He-3 in core is true): [latex]_2^3He + _2^4He \rightarrow _4^{7}Be + \gamma + 1.5861 MeV[/latex] Beryllium-7 is unstable and decays to Lithium-7 via electron capture: [latex]_4^7Be + e^- \rightarrow _3^7Li + V_e + 0.861893 MeV[/latex] This is exactly what is triggering Chlorine based neutrino detector (neutrinos from proton-proton have up to 0.42 MeV too low to trigger this kind of detector). True. We're ashes remaining from older generation of stars. He-3 can probably only two (or three) ways to be created: by decay from Tritium, or by fusion between proton and Deuterium.
-
Solar fusion, neutrinos and age of solar system
I didn't calculate from whole Sun. But multiplied by 0.34 (34%) first (calculated 60% from just core). 0.34 * 0.6 = 0.204 Yes, I am aware of no convection (according to theory/model). But I don't care about convection in this equation at all.. If you start reaction with x particles (no matter if it's star or chemical reaction at home/lab), and then it's growing to x+y after t seconds. x remain constant. y/t is rate of production. x is initial amount of Helium x+y is current amount of Helium Meaningless amount of He-4 fused further (according to wiki CNO cycle is 0.8% of energy source at the moment). I have no idea. But 2 He-3 would fuse quickly to He-4 and two protons at the beginning of life of star, so it might be meaningless.. That's why it's so rare isotope (1.34(3)×10^−6)
-
Solar fusion, neutrinos and age of solar system
Hello! Each [latex]m^2[/latex] of surface of Earth is receiving 1367 W (ignoring atmosphere influence). We know inverse-square law: [latex]P = \frac{P_0}{4*\pi*r^2}[/latex] After reversing it, we're receiving: [latex]P_0 = P*4*\pi*r^2[/latex] Distance to the Sun is approximately [latex]r = 150*10^9[/latex] meters. After plugging numbers we get: [latex]P_0 = 1367*4*\pi*(150*10^9)^2=3.8651*10^{26} W[/latex] As you can see this pretty much agree with wikipedia Sun page http://en.wikipedia.org/wiki/Sun (Luminosity on the right table) It's energy Sun is emitting every single second. One of possible fusion path is: Fusion of 4 protons to 2 Deuterium: [latex]p^+ + p^+ \rightarrow D^+ + e^+ + V_e + 0.42 MeV[/latex] (neutrino takes part of energy) [latex]p^+ + p^+ \rightarrow D^+ + e^+ + V_e + 0.42 MeV[/latex] [latex]e^+ + e^- \rightarrow \gamma + \gamma + 1.022 MeV[/latex] [latex]e^+ + e^- \rightarrow \gamma + \gamma + 1.022 MeV[/latex] Fusion of 2 Deuterium to 2 Helium-3: [latex]p^+ + D^+ \rightarrow _2^3He + \gamma + 5.49 MeV[/latex] [latex]p^+ + D^+ \rightarrow _2^3He + \gamma + 5.49 MeV[/latex] Fusion of 2 Helium-3 to 1 Helium-4: [latex]_2^3He + _2^3He \rightarrow _2^4He + p^+ + p^+ + 12.86 MeV[/latex] Sum of energies: E = 0.42 MeV + 1.022 MeV + 1.022 MeV + 5.49 MeV + 5.49 MeV + 12.86 MeV = 26.304 MeV Conversion to Joules: [latex]26.304 MeV * 10^6 * 1.602*10^{-19} = 4.2139008*10^{-12} J[/latex] Divide energy Sun has to emit by single fusion cycle to get quantity of reactions per second: [latex]\frac{3.8651*10^{26} W}{4.2139008*10^{-12} J} = 9.17*10^{37}s^{-1}[/latex] Additionally we can calculate quantity of neutrinos: [latex]9.17*10^{37}*2 = 1.8344*10^{38}[/latex] [latex]\frac{1.8344*10^{38}}{4*\pi*(150*10^9)^2}=6.488*10^{14} m^{-2}=64.88 \frac{bln}{cm^2}[/latex] Which also nicely fits with wiki page neutrino flux 65 bln. http://en.wikipedia.org/wiki/Neutrino Mass of the Sun is [latex]1.98855*10^{30} kg[/latex] Single atom of Hydrogen has mass = [latex]\frac{\frac{1.007825}{1000}}{6.022141*10^{23}}=1.6735*10^{-27} kg[/latex] Single atom of Helium has mass = [latex]\frac{\frac{4.0026}{1000}}{6.022141*10^{23}}=6.6465*10^{-27} kg[/latex] If we know mass of object, and what it's made of, we can calculate quantity of atoms. According to this website: http://en.wikipedia.org/wiki/Solar_core Sun's core has 34% of mass of the Sun. And it's made of Helium in ~60%, and ~40% Hydrogen. Phrase "so the innermost portion of the Sun is now roughly 60% helium" can be found on net and Sun wiki page http://en.wikipedia.org/wiki/Sun [latex]1.98855*10^{30} kg * 0.34 * 0.4 = 2.7*10^{29} kg / mass_H = 1.616*10^{56}[/latex] Hydrogen atoms [latex]1.98855*10^{30} kg * 0.34 * 0.6 = 4.0566*10^{29} kg / mass_{He} = 6.1*10^{55}[/latex] Helium atoms If core has 34% of mass then in outer regions of the Sun there will be: [latex]1.98855*10^{30} kg * 0.66 * 0.7346 = 9.6412*10^{29} kg / mass_H = 5.761*10^{56}[/latex] Hydrogen atoms [latex]1.98855*10^{30} kg * 0.66 * 0.2485 = 3.2614*10^{29} kg / mass_{He} = 4.907*10^{55}[/latex] Helium atoms In total we can find now [latex]6.1*10^{55}+4.907*10^{55}=1.101*10^{56}[/latex] Helium atoms Initial quantity of Helium atoms according to theories was 27.4%, so: [latex]1.98855*10^{30} kg * 0.274 = 8.1978*10^{55}[/latex] Helium atoms Time needed to fuse can be calculated by f.e.: [latex] t = \frac{Q_{current}-Q_{init}}{rate}[/latex] (Q - quantity) [latex] t = \frac{1.101*10^{56}-8.1978*10^{55}}{9.17*10^{37}}=3.07*10^{17} seconds = 9.7[/latex] bln years Why so large discrepancy from 4.56 bln years? Where is error? Abundance of Helium-4 in the core is lower than 60% as all materials are suggesting? And this graph would make Sun even more older than that: The smaller luminosity (power), the smaller amount of Helium-4 produced per second, and star must be even older.. Faster rate of production = more energy emitted to cosmos = vaporization of planets.. ps. In older version of calc, I was taking into account also lost of mass due to flares etc. but don't want to be boring you too much..
-
The Official JOKES SECTION :)
- what's a good programming language to learn?
Of course I thought about C/C++ as pretty low-level languages. (I even wrote sound card driver for C-Media PCI card 15 years ago) I meant that you don't write driver/OS in f.e. interpreted languages.- what's a good programming language to learn?
Not true. You can't make f.e. 3d game with PHP/JavaScript. You can't write drivers using high level languages. You can't make operating system using high level languages. You can't write memory monitor in Java/JS/PHP/Basic. etc. etc. There are many things that won't have sense doing in interpreted language, because the way they are working, program would have to run years to finish.. If you have to use 3rd party library written in C/C++ in some high level language to do something (f.e. render 3d graphics using CPU (pixel by pixel)), it just proves inability of doing it natively in given language. - what's a good programming language to learn?
Important Information
We have placed cookies on your device to help make this website better. You can adjust your cookie settings, otherwise we'll assume you're okay to continue.