-
Posts
7927 -
Joined
-
Last visited
-
Days Won
26
Content Type
Profiles
Forums
Events
Everything posted by Sensei
-
You can create any heavier isotope by bombarding lighter isotope by free neutrons. Actually that's a way to detect free neutrons. For instance if you will emit free neutrons to heavy water D2O once free neutron is absorbed by Deuterium, it's changing to Tritium 1H3 Tritium is unstable and decays to 2He3
-

Kramer on energy (hijacked from "What exactly is energy?")
Sensei replied to Kramer's topic in Speculations
Not anymore, since now it's in speculations. You are free to speak. Please explain - how gigantic mass of your sub-particles (10^-9 kg) can create billions time less massive particles that we all know? -
If it's really closed, mass M should remain the same, regardless of radioactive decay of components inside. Take for example (more or less closed) Earth- we have significant radioactivity in inner & outer core of Earth. It doesn't mean that Earth is losing mass with time (as long as no neutrino is emitted to cosmic space).. All (significant) energy decaying particles are carrying with them, are used to heat remaining particles. For more precise calculation, taking care of particular parent and daughter isotope, anybody can see mine signature.. (unfortunately) it's principle of radioactivity.. Einstein didn't like it, as it appears happening without a cause. It's now known that we can at least stop unstable isotopes that have exclusive decay mode through electron capture from decaying by ionizing them permanently. The first one (the lightest) isotope undergoing electron capture exclusively isotope is Beryllium-7. Beryllium-7 + e- -> Lithium-7 + Ve + 0.861893 MeV (that's what Chlorine-37 based neutrino detector is detecting the most often) I cannot agree with it. Radioactivity is decay of unstable isotope nucleus. Transformation of heavier nucleus to lighter nucleus. Even on this forum it was proposed in speculation section by Arnaud Antoine ANDRIEU in his thread. Otherwise we would have problem... If protons would start turning to antiprotons, and electrons to positrons (like Arnaud proposes), they would annihilate with regular matter.. Without any doubt.
-
Ultra high energy photons article mentions detection of gamma photons with energies above 10 EeV (16 Joules). http://en.wikipedia.org/wiki/Ultra-high-energy_gamma_ray Ultra high energy cosmic rays: http://en.wikipedia.org/wiki/Oh-My-God_particle Electron-positron pair production happens at billions lower energies (1.022 MeV photon) than these gamma photons have energy. http://en.wikipedia.org/wiki/Pair_production Ultra high energy photons (not only) are making shower of real particle-antiparticle pairs. http://en.wikipedia.org/wiki/Extensive_air_shower (so for gamma photon with >1.88 GeV, there might be created proton-antiproton pair) Pair production is real. It has been observed in Cloud Chamber the first time in 40's XX century (and scientist got Nobel price for it).
-

Kramer on energy (hijacked from "What exactly is energy?")
Sensei replied to Kramer's topic in Speculations
This mass is GIGANTIC... from point of view of particles that we know... mp = 1.67*10^-27 kg me = 9.11*10^-31 kg IMHO any sub-particle should have mass smaller than anything what we know currently. Mass of Earth is equal to sum of all masses of all particles. Mass of Sun is equal to sum of all masses of all its particles. That's pretty known for centuries. So your sub-particles must have smaller mass, smaller energy, than anything they're making when you compose couple or more of them together. Otherwise it wouldn't have any bit of sense. Deuterium has mass almost double proton. Helium has mass almost quad proton. etc. etc. Mass doesn't diminish with distance. Especially if you have it on weight and measuring its mass... -

NASA's Unexplained Spectral Line Mystery - Dark Matter Signal?
Sensei replied to IM Egdall's topic in Science News
Nobody noticed errors in video @ 1 min.. ? Fe-25?! Should be Fe-26 S-15? Should be S-16... Sulfur with 16 protons, and 1 electron left, should have approximately 13.6*Z^2=13.6*16^2 = ~3494.1892 eV ionization energy... That's at least mentioned value in http://en.wikipedia.org/wiki/Ionization_energies_of_the_elements_%28data_page%29 -
That's older than Einstein equation.. E = Q*U (P=I*U multiplied by time) 1 J = 1 C * 1 V so for Q=e 1.602e-19 J = 1.602e-19 C * 1 V (1 eV) Kinetic energy that has single electron in 1 Volt potential difference. E.K. = 1/2*me*v^2 so v=SQRT(E.K.*2/me) v = SQRT(1.602e-19*2/9.11*10^-31)=593,044 m/s
-

Kramer on energy (hijacked from "What exactly is energy?")
Sensei replied to Kramer's topic in Speculations
I thought so you're talking about something else. Neutrino needed for conservation of energy, without any doubts. Then solve your own equations for instance for me = 9.11*10^-31 kg What you will get? What will be Ee and Eg and R for such input mass? If R is constant ("distance between two extremes of Plank area") then I see no way to match experimental data.. Yes, a bit. But how they correlate? But what is it? Why are you dividing by 0? E = h * c / wavelength, where wavelength = 0? -
Thank you. I tried couple firsts and now they're loading fine.
-

Kramer on energy (hijacked from "What exactly is energy?")
Sensei replied to Kramer's topic in Speculations
Because you're giving neutrino special role that it does not have. At least there is no experiments that would show it. Am I mistaken? Sorry, but I don't understand what you even want from me.. Can you explain? Planck size area? Compton size? Planck length is 1.6*10^-35 m Compton wavelength is 2.42*10^-12 m Planck length is ~1.5*10^23 smaller value than Compton wavelength.. -
If you have object at rest, how much energy you must spend to accelerate it to v=1 m/s? Repeat calcs with acceleration from v=1 to v=2 m/s Repeat calcs with acceleration from v=2 to v=4 m/s Then with any v you wish. Even in Newton's mechanics, energy is approaching infinity the higher is velocity. That's why cars, airplanes, space ships have natural limit of speed. Their engine can't provide more energy per unit of time to accelerate vehicle even further.
-
It can be answered by analyze of what happens to electron while annihilation with positron, and production of gamma photons. They are later absorbed and emitted with less, and less energy, with more quantity... Single pair of electron-positron has enough energy to heat 1.3 billions of H2O molecules for 1o C.
-

Kramer on energy (hijacked from "What exactly is energy?")
Sensei replied to Kramer's topic in Speculations
But that's neutron decay, 15+ minutes later.. If free neutron will be absorbed by some nucleus, and final isotope will be stable, then no neutrino, but f.e. photon or other particle will be emitted.. f.e. n0 + Li-6 -> T+ + He-4 + 4.784 MeV -
-

Angular velocity & impossible situation of relativity
Sensei replied to radicalsymmetry's topic in Speculations
Can't you write single post without being rude? If forum activity makes you so much angry, you should find other hobby.. People here want to help others, not to insult them.. -
XIX century argumentation for aether was that all waves they knew were requiring medium in which wave is propagating. Waves in ocean need water. Sound waves need air or other material. etc. etc. So that argumentation was also expanded to light propagating in vacuum. To create vacuum you need to suck the all air molecules that are in hermetic container. How do you create aether by sucking regular molecules out? Is aether only in vacuum or everywhere, even inside of us and all matter? You should first create experiment that is creating aether (if it exists) in lab then come with theory. Not reverse..
-

Inversely Proportional Nesting Doll Systems and a formula for Matter
Sensei replied to Alias Moniker's topic in Speculations
Only free proton has such rest mass. More precise value is given in eV unit: 938272046 eV/c^2 (or shorter 938.272 MeV/c^2) Only free neutron has such rest mass. More precise value is given in eV unit: 939.565 MeV/c^2 Free neutron is decaying within 15 minutes: n0 -> p+ + e- + Ve + 0.78 MeV You can multiply mass in kg by c^2 to get energy in Joules, then divide by e = 1.602e-19, to get energy in eV electron Volt unit. MeV is 1e6 eV See 9.11e-31 * 299792458^2 / 1.602e-19 = 510999 eV = 0.511 MeV Actually rest mass of free electron (or positron) is me = h*fC/c^2 fC - Compton Frequency = 1.23559*10^20 Photon with at least double Compton Frequency has E =1.022 MeV and is able to create electron and positron in pair production. That's simply not true. mp/me = 1836.15 times heavier. -
It's hard to bother explaining decay branch that is happening in just 2.19×10−10% of decays of U-238... It's 1 decay per 456 billions decays that I showed. I just wanted to show counter example to your claim that half-life of daughter isotope is higher than parent isotope. You're mixing what is source. During explosion of supernova existing lighter atoms are bombarded by free neutrons. That doesn't mean that there will be created only uuh and uup etc, that will start decaying.. Somebody watching that graph might have such impression, because they're on top of it.
-
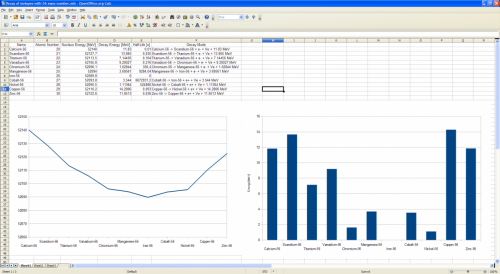
Thanks. About your observation: see Uranium. Both U-235 and U-238 (especially) have very very large half-life, counted in billion years. Daughter product of their decays have small half-life (days), and grand daughter product have even smaller half-life (minutes). Isotope Uranium-238 Protons 92 Neutrons 146 Mass 238.051 Nucleus Energy 221696 [MeV] Uranium-238 -> Thorium-234 + alpha + 4.26992 MeV Proton emission prohibited (-7.626 MeV) Neutron emission prohibited (-6.15392 MeV) Beta decay- prohibited (-0.147362 MeV) Beta decay+ prohibited (-4.47952 MeV) Electron capture prohibited (-3.45752 MeV) Thorium-234 half-life 24.10 days Isotope Thorium-234 Protons 90 Neutrons 144 Mass 234.044 Nucleus Energy 217964 [MeV] Thorium-234 -> Radium-230 + alpha + 3.67171 MeV Proton emission prohibited (-8.17297 MeV) Neutron emission prohibited (-6.19007 MeV) Thorium-234 -> Protactinium-234 + e- + Ve + 0.272928 MeV Beta decay+ prohibited (-5.51087 MeV) Electron capture prohibited (-4.48887 MeV) Protactinium-234 half-life 1.17 minutes. Isotope Protactinium-234 Protons 91 Neutrons 143 Mass 234.043 Nucleus Energy 217963 [MeV] Protactinium-234 -> Actinium-230 + alpha + 4.11231 MeV Proton emission prohibited (-5.68104 MeV) Neutron emission prohibited (-5.21991 MeV) Protactinium-234 -> Uranium-234 + e- + Ve + 2.19451 MeV Beta decay+ prohibited (-1.29493 MeV) Electron capture prohibited (-0.272928 MeV) Uranium-234 half-life 2.455×105 years Isotope Uranium-234 Protons 92 Neutrons 142 Mass 234.041 Nucleus Energy 217961 [MeV] Uranium-234 -> Thorium-230 + alpha + 4.85778 MeV Proton emission prohibited (-6.63247 MeV) Neutron emission prohibited (-6.84425 MeV) Beta decay- prohibited (-1.8098 MeV) Beta decay+ prohibited (-3.2165 MeV) Electron capture prohibited (-2.19451 MeV) Thorium-230 half-life 7.538×104 years ps. Posts #24 & #25 links don't work as well..
-
It's ridiculous to give links on forum that need your permission to view them. You should zip your files, and attach them to posts IMHO. Your graph is also unreadable - what is in X axis, what is in Y axis? I have no idea.. Axes should be rescaled to the right unit (that doesn't have exponent, like f.e. GeV instead of eV when energies are too large), and have titles. It would make sense to make graph showing different daughter and parent isotopes half-life. f.e. half-life on Y axis, and Z atomic number on X axis.
-

Kramer on energy (hijacked from "What exactly is energy?")
Sensei replied to Kramer's topic in Speculations
Mass of black-box before and after annihilation remain constant. And is 2*10^9*me kg -

Kramer on energy (hijacked from "What exactly is energy?")
Sensei replied to Kramer's topic in Speculations
Neutrinos (from unstable nucleus) are ONLY produced by beta decay-, beta decay+ and electron capture, Proton emission in proton-rich nucleus, Neutron emission in neutron-rich nucleus, Alpha decay, are always neutrinoless. Double beta decay+ or double beta decay- are occasionally neutrinoless. That are facts. Neutrino is classic old quantum theory is needed only to conserve lepton number, and to explain mismatch of mass-energy between mass of parent isotope and daughter and other products of decay. But later have been found reactions that violated conservation of lepton number. Read carefully articles before proceeding: http://en.wikipedia.org/wiki/Proton_emission http://en.wikipedia.org/wiki/Neutron_emission http://en.wikipedia.org/wiki/Alpha_decay -
Yet Another "Aether theory"... Not again.....
-
ajb said MOLE. Mole is quantity of particles or molecules. 1 mole of water is 6.022141*10^23 molecules of H2O. So it's 2 moles of Hydrogen = 2*6.022141*10^23 = 1.2044282*10^24 Hydrogens. And 6.022141*10^23 Oxygens.
-

"how to calculate Planck const at home"
Sensei replied to Iwonderaboutthings's topic in Quantum Theory
Did you do calcs? Or even better do experiment in real world electronic circuit? Article says about wavelength = 760 nm E = h*c/760 nm = 2.61374448971061E-019 J / e = 1.6315508675 eV Article's voltage drop = 1.63 V (does match) Article says about wavelength = 610 nm E = h*c/610 nm = 3.25646854455747E-019 J / e = 2.0327519005 eV Article's voltage drop = 2.03 V (does match) Article says about wavelength = 590 nm E = h*c/590 nm = 3.36685730877976E-019 J / e = 2.1016587446 eV Article's voltage drop = 2.1 V (does match) And so on so on with the rest, with little exceptions (f.e. white led with colorful plastic cover) I didn't do just calcs in thread. I have checked it a long time ago before, using colorful LEDs to check it by myself. Before even seeing that table on wikipedia (that I noticed three days ago). And drop of voltage on element is in many cases matching photon energy emitted by diode (h*f=e*U) (+ little lost). Plug diode to breadboard, attach cables to laboratory power supply, with smooth adjustment of current and voltage, set I=10 mA, and start with U=0 V, and spin it until diode is starting emitting light. Compare with wavelength of emitted photon. Done.- 40 replies
-
-1