-
Posts
7927 -
Joined
-
Last visited
-
Days Won
26
Content Type
Profiles
Forums
Events
Everything posted by Sensei
-

Solar experiment that gets 7KW per square meter of sunlight??
Sensei replied to SolarGraphene's topic in Experiments
To heat 1 gram of water for 1 C, you need to use approximately 4.1855 J energy (XIX century calorie unit) So for 0.8 L it's ~800 grams (better measure mass, it'll be more precise than measuring volume) = 800 * 4.1855 J = 3348.4 J needed for 1 C increase. If it takes your device 13 seconds to receive such increase in temperature of water, divide it by time: 3348.4 J / 13 s = 257 W (= 257 J/s) No. As you can read on http://en.wikipedia.org/wiki/Sunlight it's max 1050 W/m^2 at Zenith. Your Fresnel lens have area 0.4731 m^2 0.4731 m^2 * 1050 W/m^2 = 496 W Receiving 257 W from max 496 W possible is not bad result IMHO for home made experiment. (and we don't know at what hour you did experiment). Fix video subtitles.. If I were you I would surround water container by mirrors from all sides (buy little aquarium and cut mirrors perfectly to match its borders), and leave just little hole on top for incoming light from Fresnel lens. You might also consider using vacuum flask, or surround it by Styrofoam. -

"how to calculate Planck const at home"
Sensei replied to Iwonderaboutthings's topic in Quantum Theory
See in how many equations there is used h. You can reverse these equations. It's basic mathematics - searching for unknown y, when there is known x... y=f(x) Plug x to equation, and solve it, and you have y calculated. But in pure math, values are usually meaningless. While in physics, they're measured quantities from real world. E=h*c/wavelength so after reversing equation we're receiving: h=E*wavelength/c E = Q*U Q=I*t so E=I*t*U so you have h=I*t*U*wavelength/c I - current from ampere meter U - voltage from voltmeter t - time from stopper c - constant, but can be measured in experiment wavelength - can be measured using optics equations.. For single electron Q=e, so equation simplifies to: h=e*U*wavelength/c When U is less than 1.9 V, and wavelength is 650 nm, red LED won't emit light. If it's higher it's emitting light. Similar for green,blue,ultraviolet diodes, but different voltages U will be needed. -

"how to calculate Planck const at home"
Sensei replied to Iwonderaboutthings's topic in Quantum Theory
Of course there is many ways to calculate h, or any other physical constant.. That's how we verify whether it's correctly calculated - checking it different method. In post #5 I gave you a way to measure it and calculate using electronic circuit, with just a couple instruments like voltage meter, ampere meter, stopper, with just a few equations.. h is derived from kinetic energy of electron needed to emit photon with well known energy/wavelength.. Instead of discussion how to make experiment we're again talking about some basic things unrelated to subject.. -

"how to calculate Planck const at home"
Sensei replied to Iwonderaboutthings's topic in Quantum Theory
Iwonderaboutthings, e is elementary charge when it is usually used in physics equation. e inside of value f.e. 1e6 = 1000000 is exponent in Scientific notation.. http://en.wikipedia.org/wiki/Scientific_notation e (equal to 2.71828....) is Euler number http://en.wikipedia.org/wiki/E_%28mathematical_constant%29 Used mostly in mathematics. Three completely unrelated usages of 'e'.... You HAVE TO know which one is used at the moment.. Every scientists know it from context of equation. -

"how to calculate Planck const at home"
Sensei replied to Iwonderaboutthings's topic in Quantum Theory
Where did you see p for position? p is momentum in physics.. position is x -
I think you oversimplified this. In 1980 we had free access to hardware registers. And any program could do whatever machine allowed. Now that's job of operating system to talk to hardware. And regular programs have forbidden direct access to hardware.. You have to use OS functions to draw pixel on screen. You can't just set bit in bitplane.. This approach is required because different computers have completely different hardware, that program have no idea about.. Some people use linkable libraries for everything, usually beginners. Some people have to use linkable libraries, or dynamic libraries, because their internals are licensed, and if you would include it in your code, you could be accused of stealing.. So the same is nowadays..
-
There is known a couple exotic atoms existing for a short time. http://en.wikipedia.org/wiki/Exotic_atom Positron coupling with electron: http://en.wikipedia.org/wiki/Positronium Muon- orbiting around nucleus/proton: http://en.wikipedia.org/wiki/Muonium Antiproton orbiting around Helium-4 nucleus: http://en.wikipedia.org/wiki/Antiprotonic_helium
-

"how to calculate Planck const at home"
Sensei replied to Iwonderaboutthings's topic in Quantum Theory
Right. But you have to remember that e is Euler number, not e elementary charge. So in our case it's (what we were talking about in #5 post): https://www.google.com/?gws_rd=ssl#q=h*c%2F650e-9%2Felementary+charge -
Typical program is made of many, perhaps even hundreds, or thousands separate source files. Each source file is compiled to separate temporary object file. Then object files are linked together. If you will make change to one source file, out of thousands, compilation will finish in second. As only one OBJ file must be recompiled. Analogy might be building house from bricks - if one bricks has to be replaced because it's broken, you don't need to destroy whole house and start from scratch.. In reality it depends on particular case. If OBJ has f.e. class, and we're adding new methods or members to that class (so actually doing changes to .h header file), and it's widely used by whole application, then whole application must be recompiled. But if we would be fixing already existing method of class (so changing .cpp file), that has not been inlined, recompilation of whole project wouldn't be needed.
-

"how to calculate Planck const at home"
Sensei replied to Iwonderaboutthings's topic in Quantum Theory
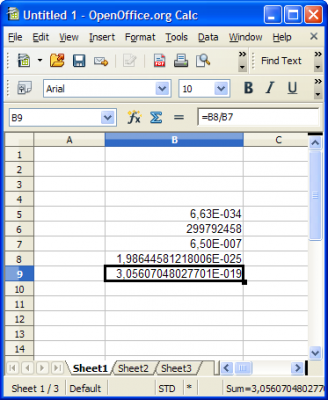
Perhaps. These values are very small, and some broken calculators might be doing calcs wrong.. No.. You are using wrong constant values. These results are also incorrect. Enter correct constants to OpenOffice Spread Sheet and you will get this: h * c = 6.62607e-34 * 299792458 = 1.9864e-25 1.9864e-25 / 650e-9 = 3.056e-19 -
Nothing. It's just a number. Planck const in electron volt units is 4.135667*10^-15 eV*s After normalization of some units to 1, you would get other answers.. You're writing wrong again. You can't write 6.24*10e18, it should be 6.24*10^18 or 6.24e18 or 6.24e+18 When there is 10 to power x, you don't use "e". f.e. 1e3 or 10^3 or 1000
-
f.e. 12 grams of pure Carbon is 6.022141*10^23 Carbon-12 isotope atoms. So if you have 1 kg of Carbon, you have 1000 / 12 = 83.333(3) mol which is 5.018451*10^25 atoms of carbon. At websites of different isotopes, different elements, different chemical molecules, you can read about their mass in unit, f.e. http://en.wikipedia.org/wiki/Isotopes_of_beryllium See column named "isotopic mass (u)" f.e. http://en.wikipedia.org/wiki/Sodium_chloride on the right you have table with row "Molar mass 58.44 g mol−1" g/mol is used by chemists, while is used by physicists. Mass of complex chemical molecule is pretty much sum of masses of atoms it's made of. H2O has 18 u = 18 g/mol = 1u + 1u + 16u (1u is approximately mass of one Hydrogen atom, and 16u is approximately mass of one Oxygen atom) if you have 1 kg of water it's approximately 1000 / 18 = 55.555(5) mol = 3.3456*10^25 molecules of water. Elements have not fully integer masses in u unit like f.e. http://en.wikipedia.org/wiki/Chlorine because typical atom has different amount of isotopes, which vary masses. Chlorine-35 isotope has mass 34.96885268 u (approximatelly 35 u) Chlorine-37 isotope has mass 36.96590259 u (approximatelly 37 u) http://en.wikipedia.org/wiki/Isotopes_of_chlorine Cl-35 has ~76% abundance, but Cl-37 has ~24% abundance, 34.96885268 * 0.7576 + 36.96590259 * 0.2424 = 35.4529375782 on website you can read average mass of Chlorine 35.45 g/mol (pretty close to above, isn't?)
-
void main( void ) { *( (unsigned int *) 0 ) = 0; } Compile and run above code... That depends on what kind of errors we're talking: about typos or real errors made by programmer..
-
Annihilation is process in which matter is converted to photons... For proton-antiproton annihilation might be much more complicated. p+ + p- -> pion0 + pion0 + pion0 or p+ + p- -> pion+ + pion- or p+ + p- -> kaon+ + kaon- (up to 9 mesons were observed in p+p- annihilation, with theoretical 13 mesons possible) pion0 typical decay mode is 2 gamma photons.
-
Your other thread was blocked because you posted it twice. Only one thread about one subject is allowed here. Such threads belongs to homework section not this one.
-

forces that interact with each other (split from critical density thread)
Sensei replied to Kramer's topic in Speculations
Because real scientists are describing observable phenomenons, not imaginary.. If I will make theory for something that I have no way to prove or disprove using currently existing experiments, it'll remain unnoticed (or forgotten) until somebody accidentally will find out experiment a few hundred years later which matches my theory.. I showed experiment how to calculate gravity at home in this thread http://www.scienceforums.net/topic/84336-if-pi-ratio-was-squared-and-98-mss-how-would-this-change-the-whole-of-science/?p=815752 Currently you don't have alternative experiment for antigravity to perform. -
I have no idea in what language it's written but A answer is almost certainly wrong. J is always constant in whole inter loop.. Doing floor( value ) has no sense for integer also. It should be used only for float argument.
-

"how to calculate Planck const at home"
Sensei replied to Iwonderaboutthings's topic in Quantum Theory
Imagine simple electronic circuit, battery with red LED (Light Emitting Diode). Single red photon which has 650 nm wavelength has energy E=h*c/650nm = 3.056*10^-019 J 3.056*10^-19 J / 1.602*10^-19 = 1.9 eV (in electron volt unit) Electron in electronic circuit that has voltage U has kinetic energy E=e*U so if voltage in above circuit will be smaller than 1.9 V, there will be too little energy to emit red photon, and LED won't shine. Q=I*t, current I you read from ampere meter, time t you read from stopper, so quantity of electrons in electronic circuit is: quantity of electrons = Q/e = I*t/e (for t=1 s, current I=1 A, it's 6.24*10^18 electrons per second) Electron is emitting red photon, and losing its kinetic energy ("voltage drop on element"). Typical LED allows 10-35 mA so it's 0.01-0.035 * 1s / 1.602e-19 = 6.242e+16 to 2.185e+17 electrons per second passing through it. (After exceeding limit I, LED will burn - too many electrons/photons per second will destroy it (if you will touch it, you can feel how hot it's becoming very quickly) ) If you will repeat this experiment with green LED, blue LED, UV LED, white LED you will see different threshold voltages at which they emit light. UV LED and white LED are similar, because in reality white LED is emitting UV photon that is later absorbed by fluorescent material and emitted in full visible spectrum. UV LED is not working with 2 AA 1.25 V (2.5 V total) batteries, but it's working with 3 AA (3.75 V). UV photon with 350 nm, needs U > 3.54 V. UV photon with 400 nm, needs U > 3.1 V. Experimentally checked. Do you now see how to calculate h? -
I think so you're misunderstanding this a bit. White color surface is good at reflecting photons at VISIBLE spectrum (approximately 400 nm-700 nm wavelengths). Black color surface is good at absorbing photons at visible spectrum. But it doesn't tell how good or bad they are at reflecting or absorbing photons at frequencies that are below or above visible spectrum. Hot black color surfaces usually are emitting light at infrared range. And are visible by IR cameras. f.e. hot carbon might still be black to us, but emitting a lot of IR photons. They need to lost energy that they absorbed from visible range photons (or other sources of energy), otherwise energy would go to infinity. If energy increases faster than material is able to emit it, it's starting changing state (solid -> liquid, liquid-> gas) Carbon dioxide gas emitting IR photons video: Metals are good at conducting heat, because (but not only) they are made of uniform atoms, which have average very short distance to each other. 1 m^3 of Aluminum has approximately 6.022141e+28 atoms 1 m^3 of Oxygen has approximately 2.69e+25 molecules.
-
Read about signed integers. The most important bit is sign bit. http://en.wikipedia.org/wiki/Integer_%28computer_science%29 http://en.wikipedia.org/wiki/Signed_number_representations
-
Byte is 8 bits. Nobody stores floating point number using a byte. Everything is in article http://en.wikipedia.org/wiki/IEEE_floating_point
-
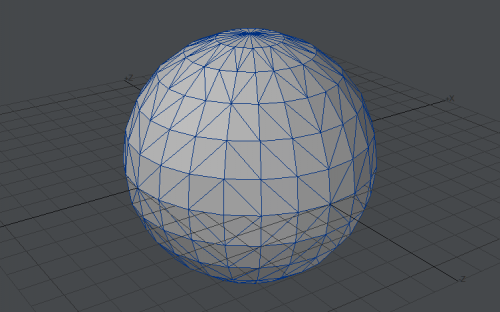
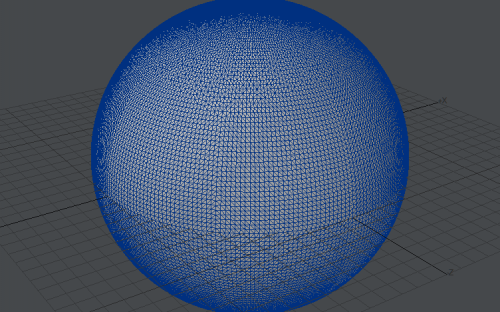
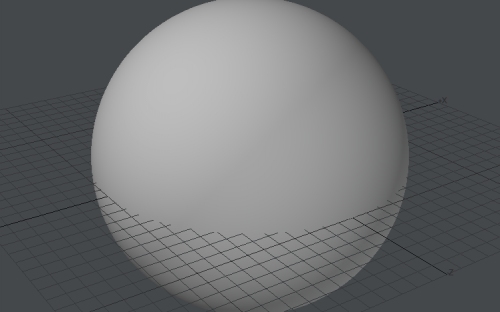
Computer graphics cards don't render anything but triangles. Sometimes gfx card driver is even simulating lines using triangle drawing procedure (one shared vertex and triangle looks like line). This saves need to create line and point drawing in hardware using transistors, for rarely used tasks. Sphere made of 528 triangles: Sphere made of ~29,000 triangles: It looks pretty smooth: Not distinguishable from perfect sphere. Concave polygon: If we order gfx card to draw polygon, it's triangulated internally: And gfx card is receiving just stream of triangles to render.. Yep. That's why I am suggesting he is extrapolating his experiences in computer programming to also real world.. I am not sure. Maybe he is suggesting that real world is some kind of simulation.
-
Simplify said he is programmer. In 3D graphics and 3D games triangle is the main building brick. The all more complex objects can be simulated using triangles. Square, or quad, are 2 triangles with uniform normal vector which share one edge. But he is extending this idea to the whole real world... without any experimental confirmation.
-
I am also finding it very encouraging. Now, they need to invest in scientists to create free (never patented) cheap, and effective (>15%) solar panels, create (or buy) solar panels creating companies, and sell them as cheap as possible to the all people on the world, or simply give them away for free (with clause not for resale).