-
Posts
7921 -
Joined
-
Last visited
-
Days Won
26
Content Type
Profiles
Forums
Events
Everything posted by Sensei
-
They looked like parachute, that later ripped apart. Some were filled with air inside and looked like water balloon (empty inside) and then tear apart. Really small raindrops after ripping apart, disappeared immediately changing to vapor, and didn't reach ground. To verify "why they look like they look" we would need to investigate how other fluids looks like when they're "sprayed" from at least couple meters height in large quantity, and recording them with high speed camera 1k+ fps with large zoom. f.e. pure methanol, ethanol These two don't have so strong hydrogen bond as water, and have significantly smaller boiling temperature (so theoretically should easier vaporize after being sprayed)
-
Raindrops don't look as everybody thinks they look like (it's rather shape of drops from faucet). In Discovery Channel there was program in which they're showing everything at 1000 fps-10k fps, and it revealed true shape of raindrops. They were flattened disks in Y axis, or rings with hole mostly.
-
Sulfurous acid, H2SO3 (H2O + SO2 -> H2SO3) Sulfuric acid is H2SO4 You can use Hydrogen peroxide H2O2 to create H2SO4
-

Do electrons radiate from electostatic acceleration?
Sensei replied to Lazarus's topic in Classical Physics
You need electron gun- two plates of metal, one with hole with + charge on it, and one solid with - charge (that's where electrons will gather). To plates there is connected high voltage. Normally electrons would move between plates. But when we will add magnets/electromagnets to bend electron beam, they will fly through hole in + metal plate, and will continue moving in vacuum. Everything in high vacuum, to minimize chance to ionize gas atoms. Then electrons can hit some medium that we will place on its path, such as metal plate. When voltage is enough x-rays will be produced. -

Do electrons radiate from electostatic acceleration?
Sensei replied to Lazarus's topic in Classical Physics
Crookes tube, that I showed above in #5 post, with metal plate on the path of electrons, with enough high voltage, will produce x-rays. -

Do electrons radiate from electostatic acceleration?
Sensei replied to Lazarus's topic in Classical Physics
Yes I know. But after molecule has been ionized it must intercepting electron from environment to become neutral again, so some electron must emit photon to do so. -

Do electrons radiate from electostatic acceleration?
Sensei replied to Lazarus's topic in Classical Physics
Crookes tube: http://en.wikipedia.org/wiki/Crookes_tube Inside this tube type there is very small amount of gas. We can see electrons path, because they emitted photons. When gas is present in the path of electrons, glow discharge is happening http://en.wikipedia.org/wiki/Glow_discharge It's used in discharge tubes: http://en.wikipedia.org/wiki/Gas-filled_tube -
When photon is colliding with charged particle, it's absorbed, and later emitted. Collision can be elastic, which means that newly created photon has exactly the same energy/frequency/wavelength as original. Or inelastic, new photon has (usually) less energy, and charged particle is accelerated. Emission of photon takes time. This feature is used by atomic clocks. http://en.wikipedia.org/wiki/Spontaneous_emission
-
Many (now) famous scientists from the past did so. f.e. Avogadro didn't said what is Avogadro constant.
-
For me it's obvious that photons going longer path will arrive later. You can see this using fiber wires (which are used by telecommunication companies, especially between different continents). Data sent at the same time, will arrive with different delay (f.e. longer pinging Australia from Europe/USA).
-
I have couple LED monitors. Let's see what are their photons polarizations. 50% photons pass through: Rotate filter -45 degree, image from monitor disappeared, ~0% pass through: Rotate fllter +45 degree. Image is fully visible, ~100% photons pass through filter. Other monitor/tv. different result: By using polarization filters we learned what was source photons polarization. When two polarization filters are one by another with 90 degree rotation, ~0% photons pass through: What is filtered out by polarization filter is reflected. In above two filters setup we can see our own reflection in black area.
-
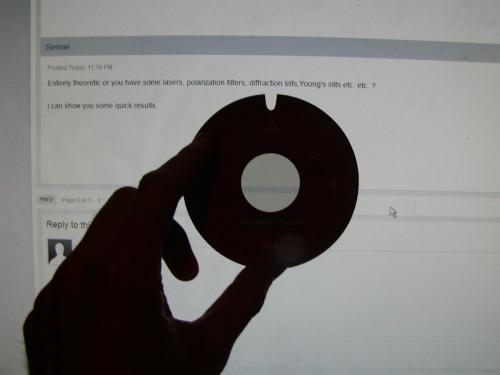
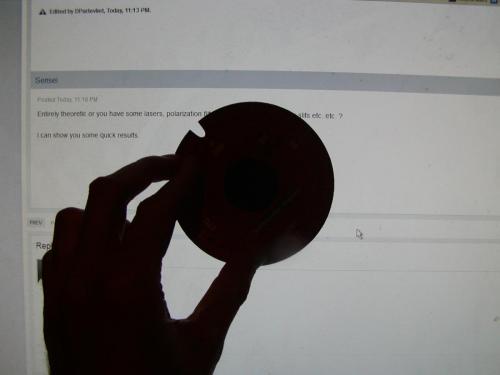
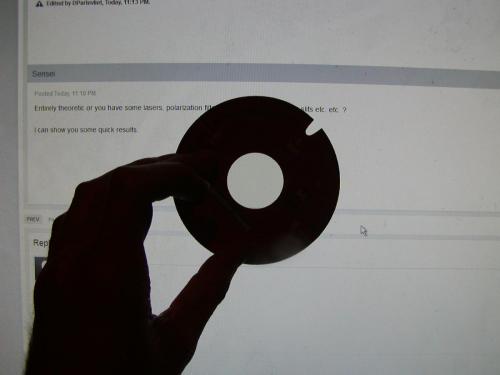
Entirely theoretic or you have some lasers, polarization filters, diffraction slits,Young's slits etc. etc. ? I can show you some quick results.
-
Are you making experiments in real, or just theoretic?
-
If there is no spectral lines difference between Hydrogen and Anti-hydrogen, and other nucleus and their anti-nucleus, we can't entirely rule out idea that some distant galaxies are not made of antimatter. After all only photons is what reaches us from distant objects.
-

Value 1.0 = Systemic Integral--0.999... = Non-Systemic Non-integral
Sensei replied to cixe's topic in Speculations
It's up to you to express yourself the way people will understand what are you talking about. Nobody will bother to try to understand somebody coming by, and writing in f.e. Chinese. You don't want to be understood, then don't write on public forum. If you do so, all your ofter will be completely ignored. Like you would never said anything. Or worser, you will be treated as person with mental breakdown. -
http://en.wikipedia.org/wiki/Two-photon_physics "Two-photon physics, also called gamma–gamma physics, is a branch of particle physics that describes the interactions between two photons. If the energy at the center of mass system of the two photons is large enough, matter can be created." Rare? Of course. Otherwise we wouldn't be here. If gamma photons would be present in large quantity, whole matter would be constantly ionized at least. In the worst scenario high energy gamma photon can split nucleus to parts. http://en.wikipedia.org/wiki/Photodisintegration
-
I have made couple devices for fast production of Hydrogen, Oxygen and Chlorine, and NaOH (because had enough waiting for slow battery rate). Mine normal rate is 1 Liter of Hydrogen per hour easily. Couple times measured. The bigger area of electrodes, the more current is flowing. But usually people are using 60 grams of NaCl and 18 grams of H2O which has volume = ~28 cm^3, so it's hard to use large size electrodes.. But I bet, he used metal which is immediately reacting with solution. If I use steel or aluminum for positive electrode, there is often no sign of Chlorine, instead electrode is dissolving. You need/should use carbon-graphite electrode for positive electrode (or better for both). 1 A * s = 1 C 1 C / e = 1 C / 1.6*10^-19 = 6.25*10^18 electrons (in theory) one electron can split H2O to OH- and H+ So there are needed 2 electrons to produce one molecule H2 1 L of H2 has mass 0.08988 g/L 1 molecule of H2 has mass 2 * 1.0078 g/Na = 2 * 1.67*10^-24 g so in 1 L there is 0.08988 g/3.3469*10^-24 g = 2.68*10^22 molecules of H2 2.68*10^22 / 3600 seconds = 7.459*10^18 molecules produced per second *2 electrons needed to each molecule= 1.492*10^19 electrons per second needed Which is 2.387 A average. I can easily go beyond this current. Today I was using 5 A to produce Hydrogen in experiment.
-
His time travel machine had failure apparently.
-

Maintaining the appearance of fixed distance in spite of length contraction?
Sensei replied to md65536's topic in Relativity
md65536, do you saw Bell's spaceship paradox? http://en.wikipedia.org/wiki/Bell%27s_spaceship_paradox -
Decimal system is just one of infinite many numeral systems. We started using it just because we have 10 fingers. If we would have 4 fingers in hand, we would be using octal system, most likely. And whole nonsense you would have to throw up through window.. It's easy to imagine that on other planet in the other galaxy there are intelligent creatures using 2,4,5,8,10,12 or whatever else numeral systems. PI in binary system will look (at least a few digits that I converted): 11.0010010000
-
Magnets and electromagnets are used to bend path of electrons inside of discharge tube, vacuum tubes, cathode ray tube etc. It's used in CRT obsolete televisors, and analog oscilloscopes.
-
??? Do you even know what is superconductor? Electrons in circuit have some kinetic energy. When they flow through conductor they're giving material part of their kinetic energy, and heating that material. We read lost of kinetic energy of electrons as drop of voltage. When metal used for wire is receiving too much kinetic energy from flowing electrons it's melting and circuit is broken. This is basically what happens when direct current is too high (I>Imax). Electrons flow through conductors easier than through dielectric, but it doesn't mean that they cannot flow through them entirely. When electrons is too much and have too high kinetic energy, they will break through dielectric, if they won't find easier path through conductors.. Air is good dielectric, but after using couple thousands volts, spark is flowing through oxygen and nitrogen ionizing them and producing plasma and electrons find way through dielectric. Superconductor is not behaving like that. Electrons can flow without giving their kinetic energy to superconducting material, so closed loop of electrons can flow "forever". If it's placed on top of regular magnet, it's levitating (because electrons are in constant movement inside of superconductor). Modern smartphones, cpus, highly integrated circuits, don't melt just because direct current (read it: quantity of electrons in unit of time) flowing through them is quite low. Pass higher I than their Imax and they will be broken, some weak path in them will melt..
-
To produce biofuel vegetables, bacterias, algae need to absorb CO2 first to grow up. Then we will burn fuel, releasing CO2 back to atmosphere. Output is equal to input. Unlike fossil fuel. Organisms that produced it absorbed CO2 hundred million years ago, and releasing it now will change climate dramatically. Humans should learn how to not waste energy in the first place. But not by doing such crap ideas as banning traditional light bulbs (which stinks corruption and conspiracy for a mile), but real things like f.e. completely free of charge local buses and local trains. This would encourage people to leave car at home. Or not buying it at all. If bus can hold 50 people, it's 50 people that didn't have to drive their cars and burn fuel, each independently. Bus is using 25 L/100 km, regular car f.e. 5 L/100 km, 50 cars/people * 5 L = 250 L of fuel total. So 1 bus is saving 90% of fuel used by all these people straight away! The main problem is that governments are earning a lot of money from excise for fuel. And the more people drive, the more money they are receiving. Impossible to break circle.
-
"Extra credit" http://en.wikipedia.org/wiki/Extra_credit I understand as special work for somebody who has knowledge beyond the rest of class, when he/she exceeds what they learn in class.. Your questions suggests you're not the right person for extra credit..