max.yevs
Senior Members-
Posts
130 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by max.yevs
-
ah right... that's what happens when you go from step1 to step 2 in your head...
-
lol sayonara, nice sarcasm. but i agree fully, its way too viscous; maybe one or two molecules on the surfaces of the drops get through but theres another consideration- who has the hiv, man or woman? given that hiv almost always spreads to the receiving partner, we guys have nothing to worry about. in fact chances of getting hiv for a man are at most .065%, .5% for a woman.
-
Sorry, I didn't mean to be rude in that post. I would edit it if i still could. But you have to admit halogens are awesome. They're the most reactive elements, save for oxygen. Except I don't like fluorine and astatine. You think rubber seals are a problem for bromine? Fluorine will eat through glass and plastic. And even its salts are poisonous. Astatine- well I'm sure only a few people in the world have handled it, its the second most radioactive element after Francium. But chlorine, bromine, and iodine- they're the perfect trio. All very corrosive, one gas, one liquid, and one solid. Chlorine i've dealt with many times, it doesn't deserve half the bad rep it gets. Iodine i've made only once, but it was very nice. Reacted with aluminum scraps without even asking it to. Bromine is actually the only one i've never handled, its corrosiveness can be hazardous. Although the liquid BrCl is even more corrosive. What was really good about this experiment is that I have found a decent way of making monohalide interhalogens. This includes BrCl, IBr, and ICl. The ones with fluorine are not only too dangerous, but wouldn't work. Fluorine hydrolyzes completely regardless of solubility.
-
Do you know why old people run to stay young? As they approach the speed of light, time dilates so they age slower.
-
i think the real problem is recreating the person. And bring him back to life. They already have something similar for simple objects, a 3D scanner and a 3D printer. This is not only a teleporter but a clone machine! And it does not even have to be light, even simpler just an a signal over an internet connection.
-
wait a sec.... say there's a beam of light... traveling at c, speed of light ther's two guys, one is standing still, and one is traveling at 0.5c know we now light always goes at speed 1c. so relative to the second guy, shouldn't it be 0.5c? or if its 1c relative to him, shouldn't it be 1.5c relative to the other one? I think that speed of anything, even light, should be taken in relation to earth, because even though its not completely stationary, its speed is insignificant compared to c.
-
recently i was thinking, if you were going at the speed of light, and you shine a flashlight forwards, would it be going at double the speed of light? of course i found out that no it can't. but this leads to an interesting modification. If you're going forward at the speed of light, and you shine a flashlight behind you, how fast would the light be moving? at 0 mph or still at the speed of light?
-
Ah ok poobah, yeah i realized its csc right after i posted the first time [math]2csc(x) - 1 = 0[/math] [math]{\frac{1}{sin(x)}} = 0.5[/math] [math]sin^{-1}(x) = 2[/math] [math]x = sin(2)[/math] [math]x = no solution[/math] as you can see then there are no solutions (since sin(x) is 1 at max.)
-
ah ok... yeah that is what i basically what I had in mind, a "ball of bright" well since this question has been explained, how about an unrelated one: if you shine a lot of light onto a reflective surface, would the reflective surface be pushed back by the light? From the photons hitting and bouncing off it?
-
ok, ok, we're not all advanced physicists you know... yeah i just picked that explosive because it has the highest detonation velocity... it's hard to say what the velocity of kerosine + lox is. i actually agree that you can't go at the speed of light, however I think you can go at 99.999999% speed of light. Because: say you're going in a rocket in space (for no friction or air resistance). Accelerating to a certain point would be simple. However, as you approach the speed of light, the reaction in your rocket slows dow due to time dilation. If you could go at the speed of light, so the kerosene and oxygen would stop reacting. The temperature of your whole ship would drop to 0K. But its ok, humans on board will not even feel the temperature drop or anything strange. To them, the kerosene and oxygen are still reacting. Here's a sketch below. As you try to accelerate to the speed of light, the rate at which you accelerate approaches to zero. which is why you would need infinite energy to get to speed of light. But it will be simple to get to 99.99% speed of light. The flight airspeed record is almost 1/three million the speed of light.
-
ok, i should rephrase that. No matter how long it will take for the people on earth to see you again, for you it will feel like no time has passed. One moment you're accelerating to the speed of light, then you and your clock and the person sitting next to you are all frozen, and the next moment you're landing on earth. You would have aged 16 minutes less.
-
i know something that can go 1/30 the speed of light: a rocket with a large octanitrocubane booster strapped to its back (it has the highest r.e.f.)... although i suppose if the nozzle is 1/30 the size of a 1:1, it can go the speed of light. but that's kind of adventuring off topic
-
i realized recently- light travels at different speeds through a vaccum, air, water, glass, etc. which is why optical lenses work... if you were to shine light through a very dense, yet clear compound, could you actually see the light progressing from side to side? and would it be like after you turn off the light it would still keep shining for some time?
-
You'd still be standing still! Imagine this: you're traveling in your car at 200 mph... a guy is waving his hand on the side of the road... however you can't see his hand move back and forth because its all a blur... which translates into he's waving too fast for you... but really he's not waving very fast, just slowly waving his hand. So your reaction must be very slow. If you accelerate to the speed of light, he is still waving at a normal speed, but all your reactions and clock and everything are slowed down to zero. You are basically frozen. (Actually your car's engine is also frozen) so for example, if you drive a rocket ship at the speed of light to the sun and back, the trip will be instanteneous. It won't take distance/(speed of light) time, it will take 0 minutes. here's a good site, although its kind of made for kids really? what would they do it with? light doesn't just mean like flashbulbs, it means any part of the electromagnetic spectrum...
-
I would think that ammonium is just slightly more reactive than hydrogen in the metallic sense, i say so because ammonium ions usually exist in equilibrium with ammonia and hydronium ions. I think it is safe to assume that most of the things an acid will react with, an ammonium salt will also react with, although less violently... but you might want to wait until you get some more responses
-
i suppose that's a good way to travel at the speed of light/ teleporter say you need to get to the store 12 miles away: jump into a black hole which will convert your mass into pure energy, fiber optic cables take the light to a white hole, where that pure energy is converted back into you! and then you're at the store! by the way, to go back in time: put a rocket on the world and make it go very fast, while you stand still. If it goes at half the speed of light, then you'll be able to move twice as fast as them. You'll age twice as fast. If it goes at the speed of light, they will be standing still, frozen, while you'll be moving at normal speed. Faster than the speed of light? They would all be walking backwards, as if you clicked the reverse button. But that's impossible. Going forward in time is easier. Accelerate in your super fast car. If you are going at half the speed of light, then for every day you and your clock experience, the world will experience 2 days and move twice as fast. You'll age half as fast. If you're traveling at the speed of light, then the world will see you as standing still, frozen. If you're traveling faster than the speed of light, you'll actually be getting younger in your car and reverting to a kid. But that's impossible. Although traveling at the speed of light is theoretically possible, traveling faster isn't, because all atoms and physics are based on the speed of light. A more realistic way to go forward in time is to cryogenically cool yourself for a couple years with liquid nitrogen and hydrogen sulfide.
-
in terms of reactivity with an acid, one whould have to suppose it would be right above hydrogen... but there's the question of ammonia vs. ammonium..when reacting with non-acids. for example, when reacting with chlorine, hydrogen would probably be more reactive... because ammonia + chlorine does not make ammonium chloride... (it makes dangerous explosive NCl3)
-
good question, idk i googled it and each link is about some settlement home in new york or something... but thanks for the tip, if not CAB, i'm sure there's something similar to it Actually i just realized, i can be pretty confident that all of those first 8 are legal, they're used in labs all the time without any special permission... 9 i would assume is also legal since its no more poisonous or corrosive then one of these: 1,2,3. Number 8, ammonium nitrate might be questionable since its the basis of the infamous terrorist explosive, but I don't like it too much anyways. And that link I gave turns out to be chemicals being transported. so i think unless I put any one in danger or anything like that, I should be good. \\thread closed
-
yeah, nitric acid works on a whole different level then most acids... supposedly only in cold alkalis/alkali earth metals it will liberate its hydrogen.. although I suppose with a base it would probably react as expected- i.e. [ce]NaOH + HNO3 > H2O + NaNO3[/ce] and doesn't NO quickly oxidize in air to NO2 hermann? So my bet is that its not much safer then NO2
-
oxidizers are always a good choice, although dangerous... after all, what is acid? HCl = hydrogen(a poor reducer) + chlorine(a good oxidizer) if you're trying to find a good acid that may be at your home, consider something like "the Works" i use it as if it was pure 20% HCl because basically its pure 20% HCl with a little blue tint... and its pretty cheap, 2$ a liter... you can also try a hardware or pool maintainance store, they probably have some HCl of good concentration. if you're trying to make it yourself, that may be harder... i suppose you could electrolyse a salt or something, but not without some hefty equipment. But be careful!
-
i don't know, all the halogens reacts violently with aluminum - after pouring the couple drops I had on a piece of foil, it started fizzing just until the brcl ran out... but it is very hard to a gas... and chlorine is slightly more reactive then bromine monochloride... but there's a special danger to bromine in the fact that its liquid- i mean aluminum will never burn in oxygen, right? but if you use liquid oxygen, the aluminum into sparks. my whole idea in this experiment was to try to make a very corrosive liquid- if not bromine, then brcl... which if you look at the first post i planned to cool it from the beginning. i know that like bromine left in a bottle, its vapors can eventually destroy the cap if its rubber... but i used brcl after maybe 10 minutes, as soon as it condensed. I wouldn't think of storing it! and by the way, that gas is at about normal pressure... in any case, i realize i shouldn't have posted those pictures here (or maybe even this whole experiment). i wanted to show how cool brcl is, not to get everyone worried... I like the way they did it in 1950s. Look at the picture on the bottom, he's holding NO2/N2O4 between 2 glasses... nobody is yelling: what if he knocks them over? And they're giving you easy instructions on to how to make some at home, without excessive warnings. Why? Because they know a glass full of NO2 wont kill you. A glass full of chlorine won't kill you. Because if someone accidently releases some, they wouldn't put their face right up to it and breathe it until they died. It's a pretty interesting article by the way, find it here, its 3 pages. I wish my popsci subscription still had pages like these. But times change, people are afraid of 'dihydrogen monoxide'. Sometimes i wish i lived in 1950s America.
-
yes i know that which is why i'm trying to make sure that it isn't illegal...explosives or something, yeah obviously that's illegal. and on second thought i realize this was a stupid post since laws for this vary everywhere. i just got a little freaked out after reading that hazmat thing.
-
After looking at the Hazardous materials sticky, i realized that a lot of the things people make might actually be illegal. Does anyone know about the legality of these or other chemicals in the U.S. (or other countries)? Assuming they are all used only in good natured experiments... 1.)Chlorine 2.)Bromine 3.)Iodine 4.)Hydrochloric Acid 5.)Sulfuric Acid 6.)Nitric Acid 7.)Ammonium Nitrate 8.)Nitrous Oxide, Nitric Oxide, Nitrogen Dioxide 9.)Non-fluorine interhalogens 10.)Any other materials commonly mentioned here I know for sure most explosives are illegal (i.e. nitroglycerine)... i know drug precursors are watched (i.e. I2)... i'm not sure where things like model rocket engines and thermite fit in...and ammonium nitrate in itself is a weak explosive. I saw something about chlorine here, but it says only if compressed or liquified... and montreal protocol only deals with chlorinated fluorocarbons, not chlorine itself... I'm sure if I do accidently use something illegal I could explain that I was doing it for the good of chemistry, but just in case...
-

Can someone tell me what im doing wrong when completing the square?
max.yevs replied to anikan_sw's topic in Mathematics
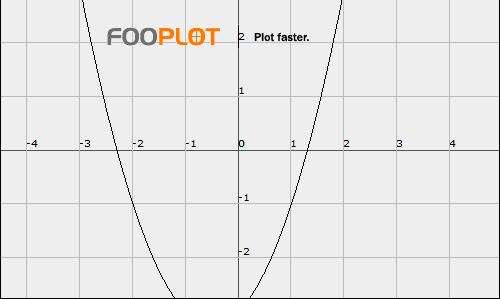
no cameron, sorry [math](m-1.73)(m+1.73) = m^2 - 1.73^2 = m^2 - 3[/math] but he was factoring [math]m^2 + m - 3[/math] you can't solve it by simple factoring because 3 only has two factors: 1 and 3; and the difference between them is 2, but the coefficient of m is 1, not 2. the roots are irrational. completing the square is impossible. only thing left to do is use the quadratic formula: [math]{\frac{-1 \pm\sqrt{1^2 - 4(1)(-3)}}{2(1)}}[/math] [math]{\frac{-1 \pm\sqrt{13}}{2}}[/math] which calculated gives [math]m = -1/2 \pm{\frac{\sqrt{13}}{2}}[/math] so, [math]m = 1.30277563, -2.30277563[/math] And for all of you who got other answers: when you complete the square all the terms and constants have to stay on one side. Below is a picture prooving my answers... (fooplot) -
oh, theo, nitric acid does react with copper, quite violently i might add... it seems like it would not because copper generally does not react with acids, but nitric acid is no usual acid- it usually is not even willing to liberate its hydrogen. [ce]Cu + 4HNO3 -> Cu(NO3)2 + 2NO2 + 2H2O[/ce] and apparently [ce]NO2[/ce] is very toxic i've never handled nitric acid, but here's a video