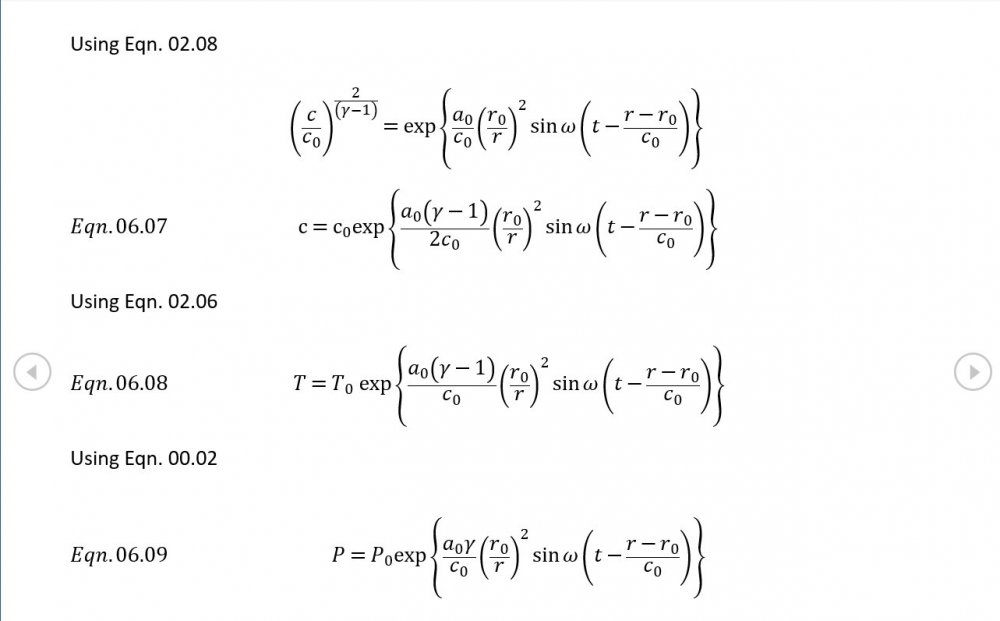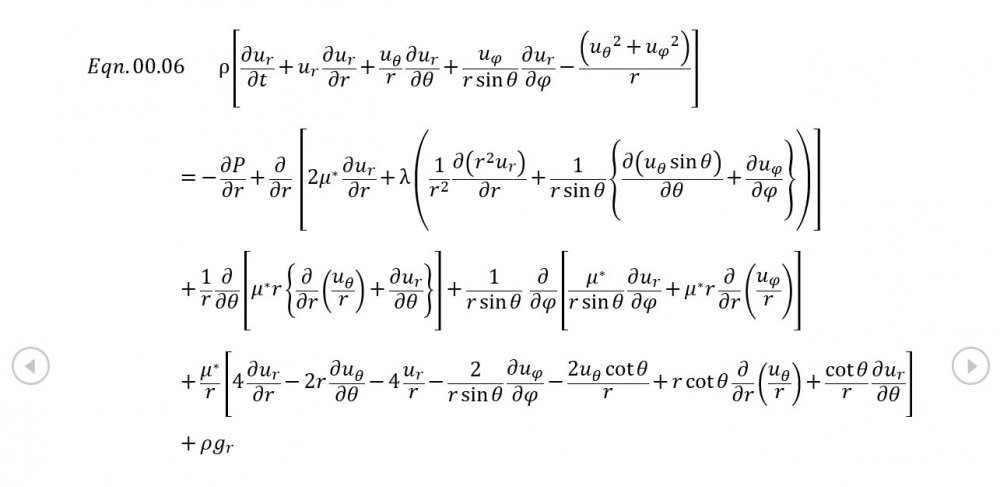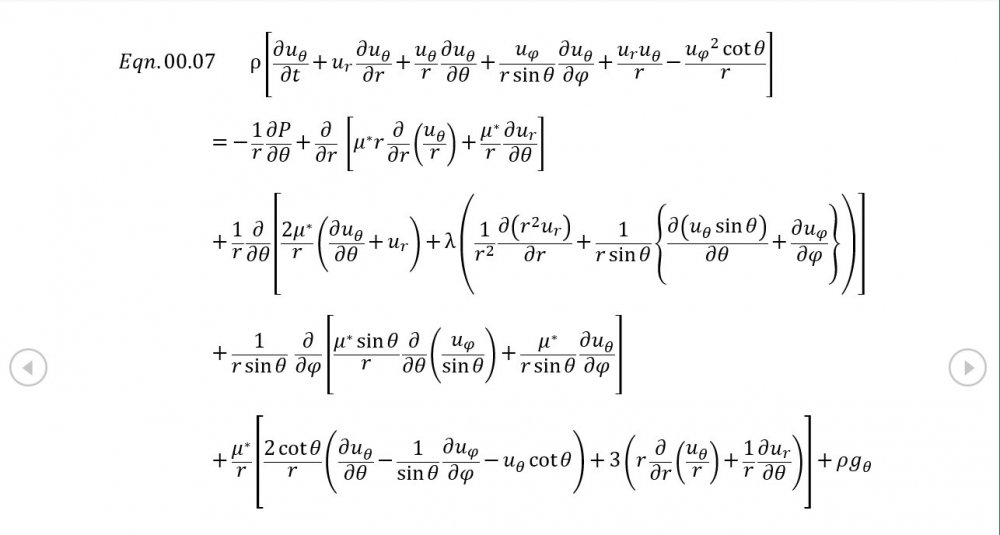Everything posted by sethoflagos
- The Nature of Time
-
The Nature of Time
Because all the energy that built the past universe was consumed in constructing the present, and the future is no more than a set of alternate building plans awaiting selection (wave function collapse if you prefer) and availability of materials for construction. Just an interpretation. Consider observing a supernova inside some huge stellar nursery from not too close but close enough to study in reasonable detail. After the early fireworks have subsided we may expect to see a growing bright edged circle as the surrounding dust is progressively illuminated. Is it unreasonable to perceive the arrival of intense radiation at that bright edge as being experienced simultaneously around the entire perimeter even when it's several light years in diameter? And what exactly is going on at that edge other than a colossal number of particle interactions being randomly chosen from an even more colossal number of possible interactions. It may even be viewed as a phase change, but in addition to a change in physical properties it's a condensation from causes to effects. Or a condensation of an unobservable, abstract 'many worlds' future into a single throughly deterministic past with the present being a thin slice of quantum fuzz between the two. Again, just a personal visualisation to help get my head around it. Perhaps, the ambiguity of 'now' for different observers is simply an SR induced form of observer bias. Not one of them is able to observe a causal reversal, and to me, that seems to be the critical issue. Is this a reasonable view?
-
The Nature of Time
I think one reason many people have difficulty getting their heads around our current concept of measuring time is that it is so abstract. What is it that distinguishes one person's 'tomorrow morning at 8am' from another's? There's very little to cross-correlate with, and if the message came by snail mail then somebody might be waiting a long time before the other shows up. Compare this with an older, more human kind of clock. Granted it lacks the accuracy of a modern atomic clock, but it does have one crucial property that modern time-keeping has lost - an explicit chain of causal links that no observer can dispute. Some might disagree on the exact timing of Eshbaal's tenth birthday, but no one in their right mind would imagine that Moza begat Ner. There are many other historic examples such as the succession of popes or Egyptian pharoahs. Perhaps the best modern example of is the geological record with its various epochs, each marked by a distinct global change in environmental conditions, and each subdivided by a detailed succession of marker fossils. When J B S Haldane answered the query of what might challenge his belief in evolution, his response of "Precambrian Rabbits" appealed not so much to the 500+ million years difference in dating (who can truly comprehend such a non-human time period?), but the obvious dislocation in an established causal chain.
-
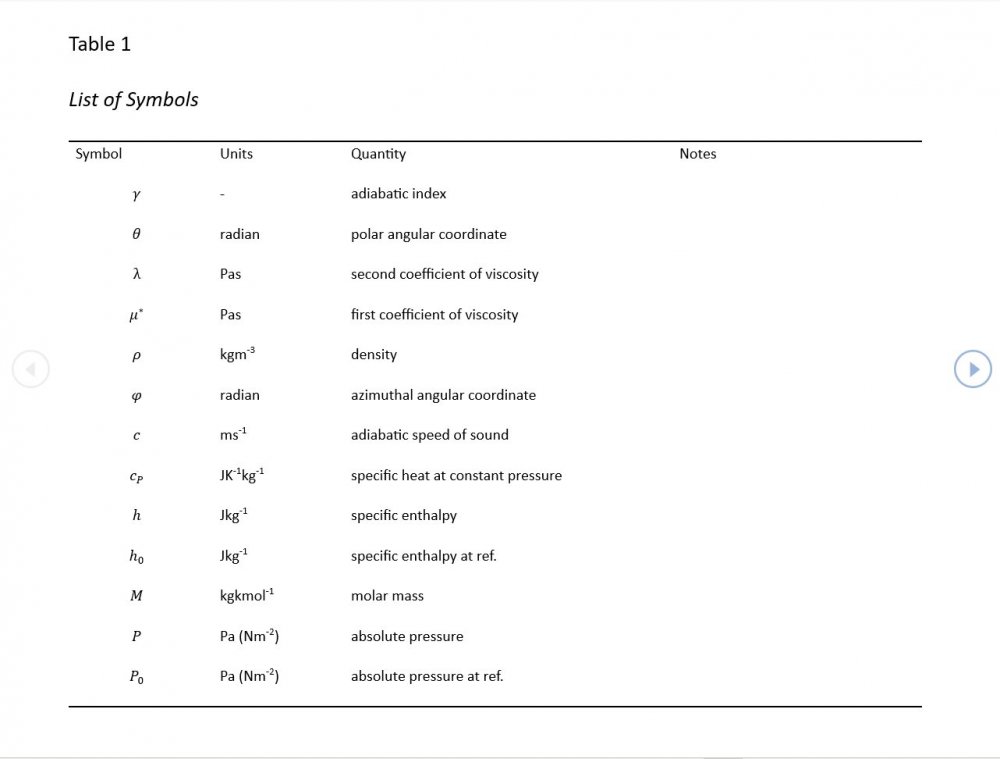
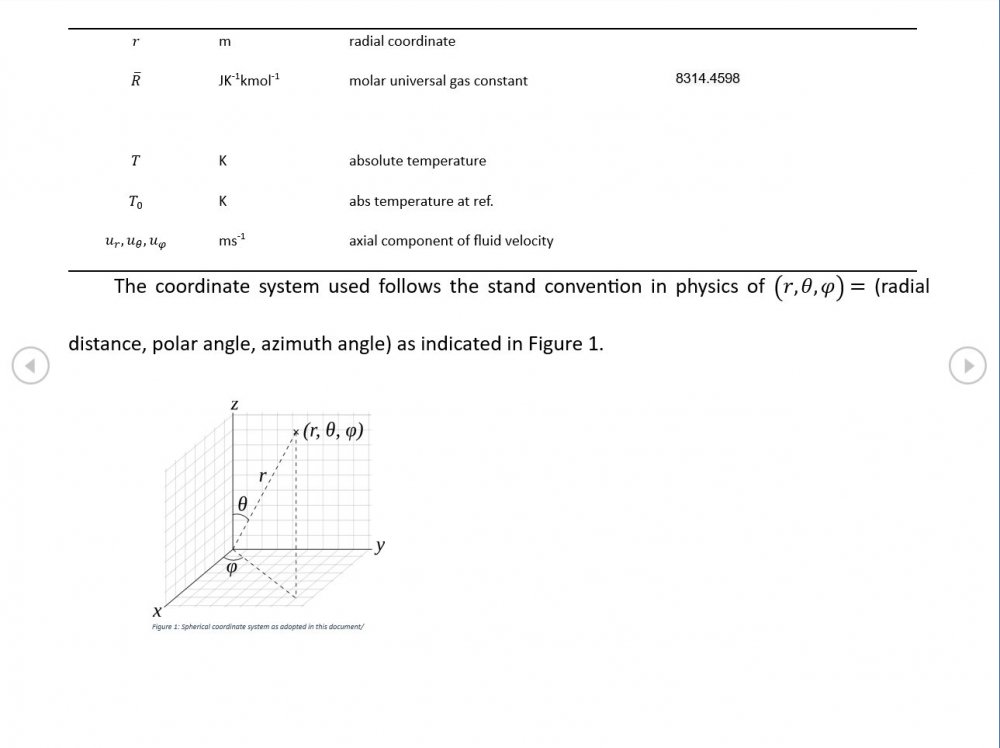
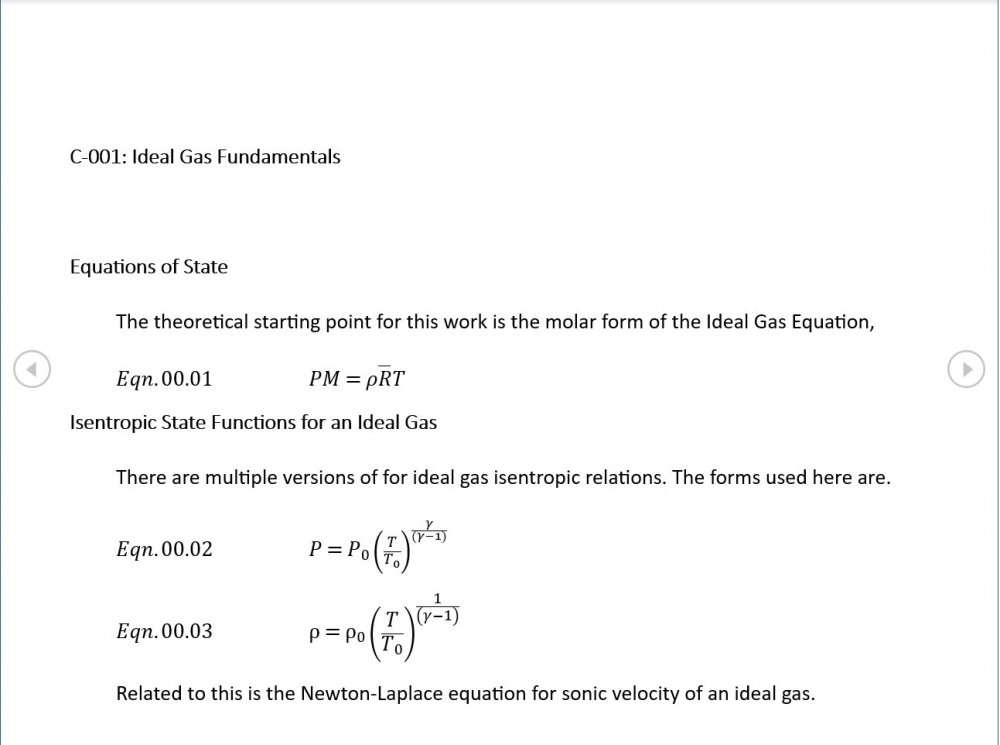
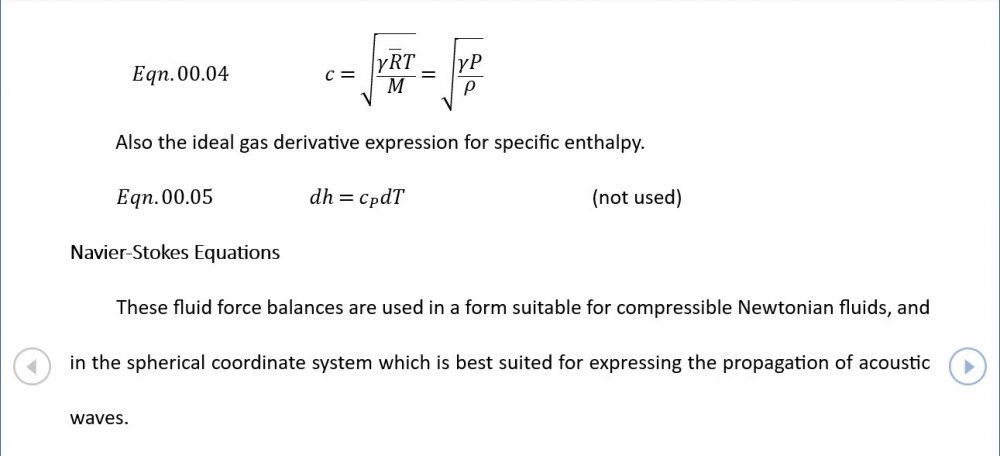
Acoustic Waves in Air with Variable Sonic Velocity
I was able to download the full text of Hansen's "Fundamentals of Acoustics" 4th Ed. It's written in a language I can follow and I've attached a sample chapter. Is this likely to guide me in the direction you're recommending? Fundamentals of Acoustics 4ed-Cap5.pdf
-
Acoustic Waves in Air with Variable Sonic Velocity
Thanks for the reference - that one's a keeper and gone straight into my acoustics library. I'm just focussing on the forward wave for now as that generates forms that are easily compared with standard reference material. If I include the reverse wave on an equal footing, I get a standing wave of sin(wt)cos(w(r-r0)/c) form. I'm not 100% sure why. Perhaps this is where I start having to take a serious look at impedance.
-
Acoustic Waves in Air with Variable Sonic Velocity
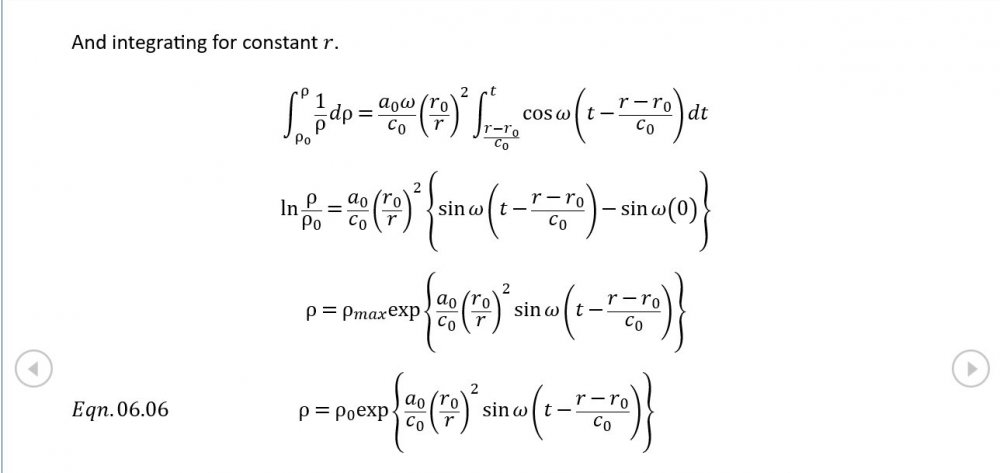
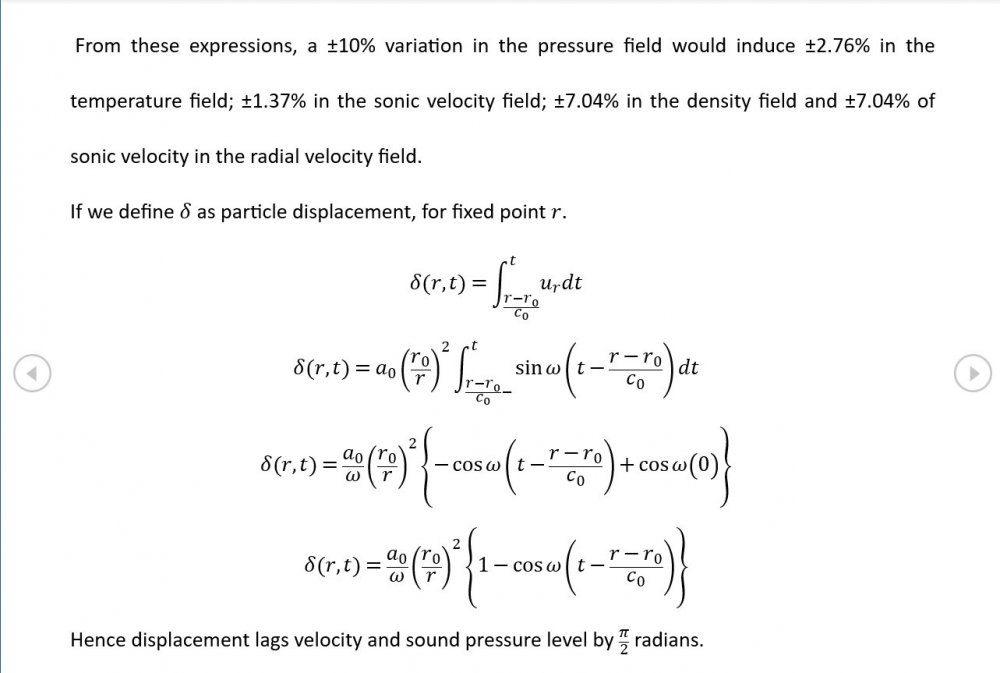
Amendments to the previous update plus preliminary approximations to all relevant fields. I really could do with someone (@Mordred) to put my use of Eqn. 06.05 under the microscope. The results that follow look credible at least as approximations, but ....
-
Acoustic Waves in Air with Variable Sonic Velocity
Good point but not insurmountable I think. The really scary challenge is that the sound output propagates back up the instrument to the mouthpiece where it acts as a high gain servo-assist on opening and closing the embouchure in sympathy with the output waveform. The output largely determines the input so there is absolutely nowhere to hang a set of initial boundary conditions unless you start from silence and integrate through all the note establishment phase.
-
Acoustic Waves in Air with Variable Sonic Velocity
Well there's the rub. As r approaches infinity the wavefront tends toward planar. Essentially I simply lose the 3rd term (2cu/r) from the ODEs and I have a planar wave. It's a little trick I found to study standing waves in low angle conical to concentric tubing. Btw I really appreciate the time you're spending on this. I know you've many other matters to attend to.
-
Acoustic Waves in Air with Variable Sonic Velocity
Shucks, missed the cut-off. r within the trig functions should read r-r0 of course.
-
Acoustic Waves in Air with Variable Sonic Velocity
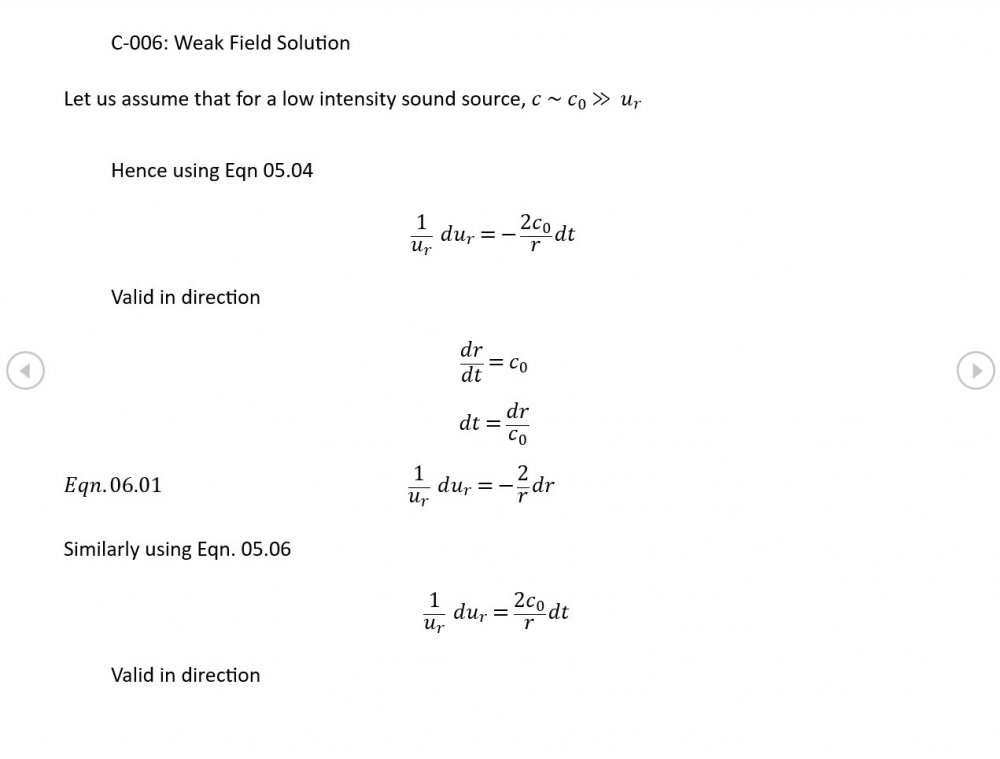
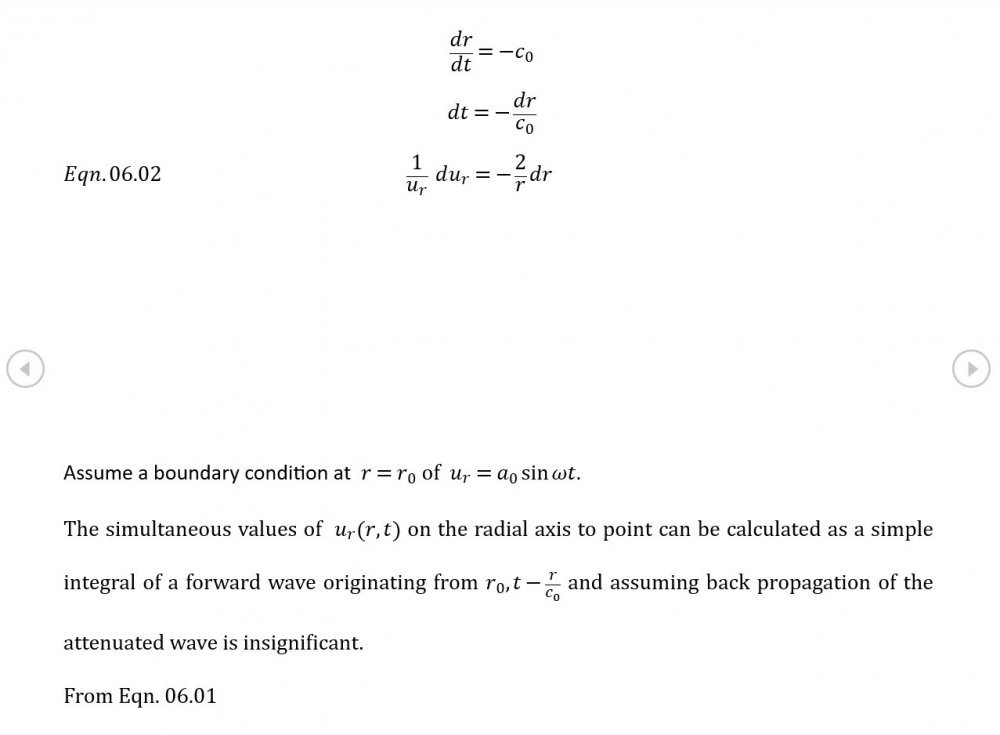
Sometimes so would I. I've worked hard all my life and set aside many topics that interested me because I could spare the time. Now that I have the time, I really would like to produce something academically worthwhile before I kick the bucket. Anyway, some weak field results hot off the presses
-
Acoustic Waves in Air with Variable Sonic Velocity
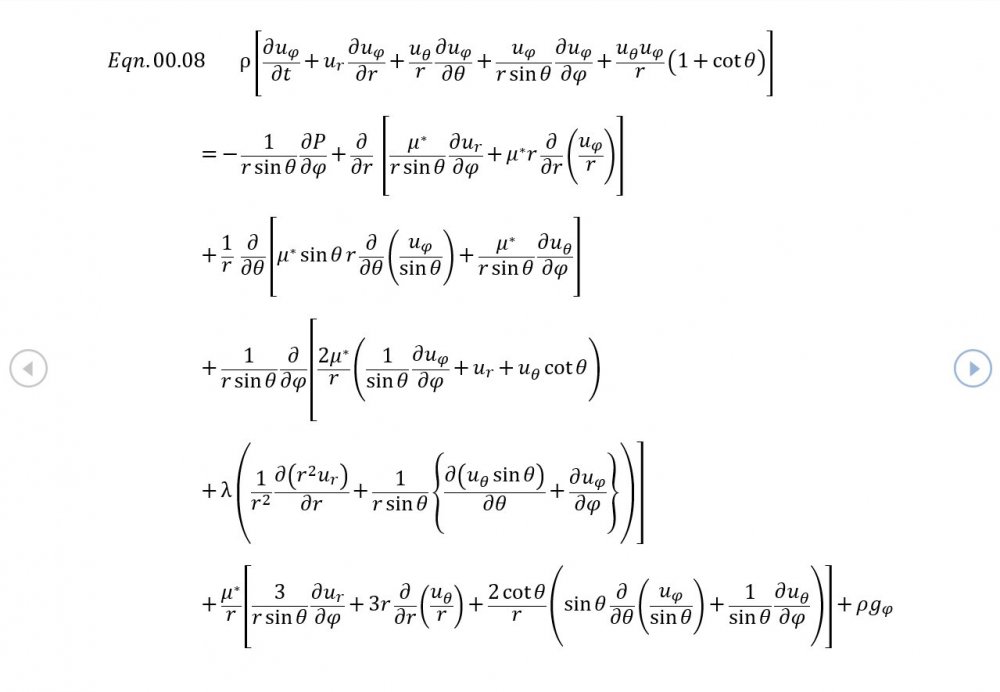
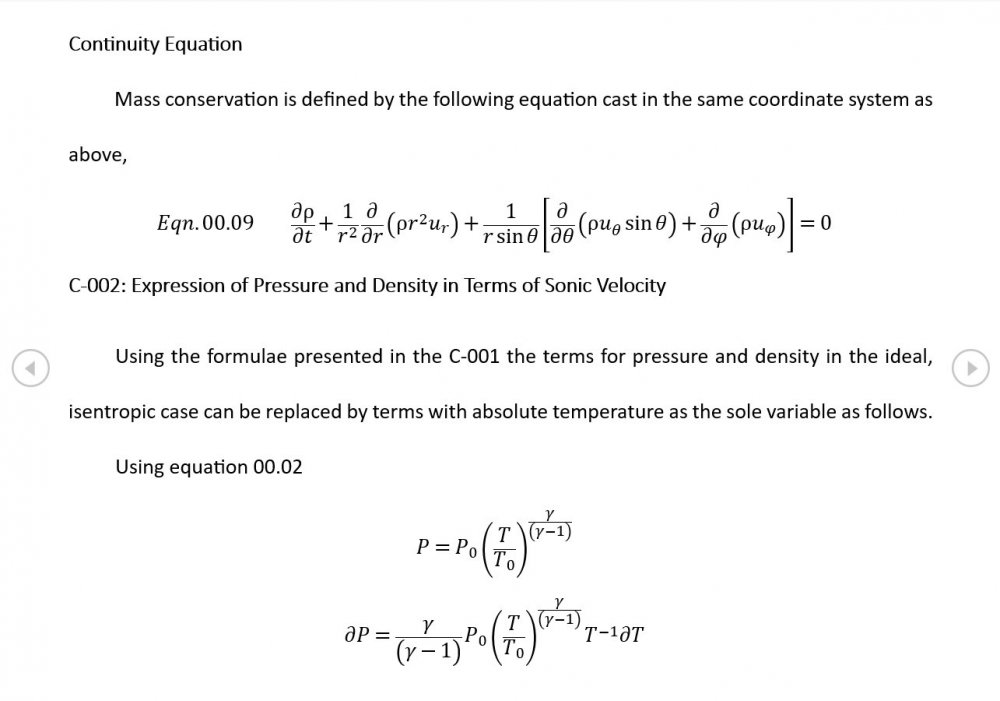
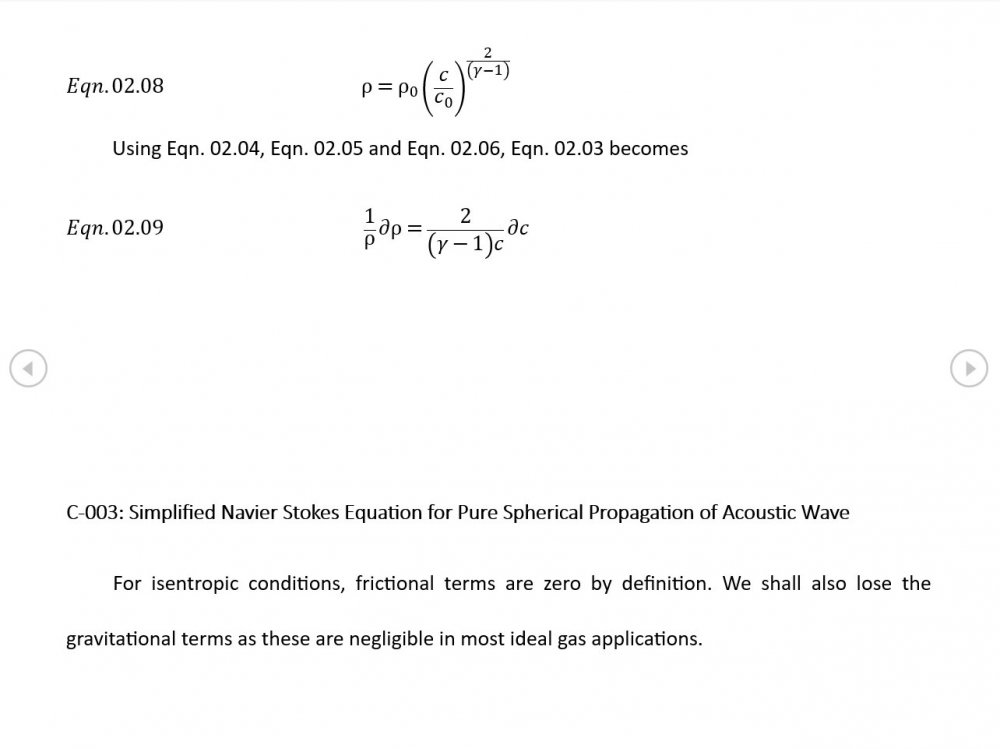
Thank you for your intervention - I appreciate it +1 Can we put the trumpet stuff to one side? It's what initially got me thinking about this topic but the OP is about building the correct general mathematical framework. The rest of your analysis is correct though the introduction of temperature via the Equation of State now needs isentropic conditions to be established via Eqn 02.02, and the introduction of a variable sonic velocity calls in Newton Laplace (Eqn 00.04) so there's 7 independent equations for 7 variables . The choice of spherical coordinates certainly helps eliminate a lot of terms that might otherwise be awkward.
-
Acoustic Waves in Air with Variable Sonic Velocity
It was based on Eqn. 02.06 given in the document posted above complete with a full derivation from thermodynamic fundamentals. Suggest you try reading it before you make any further inflammatory statements.
-
Acoustic Waves in Air with Variable Sonic Velocity
In the absence of a well developed mathematical model, these questions cannot be quantified with authority. You seem to be putting the cart before the horse. The eleven year-old trumpeter in my avatar was already getting regular paying gigs, and he says 'Citation, Please'. Or words to that effect that I'm not going to repeat in a public forum. Newtonian gravity is a far better approximation for anything you or I are likely to experience first hand. I guess this has been answered by default.
-
Acoustic Waves in Air with Variable Sonic Velocity
It's in the 'Speculations' section. If there was a citation it would be a speculation would it. Do you have anything to say on the meat of the OP or are you happy just sniping at the peripheral stuff?
-
Acoustic Waves in Air with Variable Sonic Velocity
Being 1 metre downwind of a trumpet bell will put you in the middle of a soundfield oto 1 m2. And no, the proportion of energy input that becomes acoustic energy is a matter of a musician's skill among other factors. For trumpet players above a certain standard, the kinetic energy of the DC component of flow is much less than that of the AC component. These issues are way beyond the groundwork I presented in the OP. Could we get back to that? Btw your 174 dB is effectively the sound pressure level at source ie somewhere inside the pipes which are typically 12+ mm diameter. There's a scaling factor of some thousands down to the sound pressure level at 1 metre. And I'm working with peak values not rms so 10 kPa at source will typically become oto 20 Pa at standard distance.
-
Acoustic Waves in Air with Variable Sonic Velocity
10 kPa, 8 K, 2.4 W, 120 dB seems to give a consistent picture as back-of-envelope calculations go. It's your 174 dB which is way out in left field. That's instant pulmonary embolism, burst lung zone.
-
Acoustic Waves in Air with Variable Sonic Velocity
I can never figure those things out. dB is for electricians. On a practical level, I've plugged a water manometer in the side of my mouth and registered about a metre water gauge when I'm playing a little more than reasonably loudly. If someone stuck their head within 1 metre of the business end, they'd be in some discomfort. That ties in with the 'threshold of pain' in the tables at 120 dB. I'm not sure whether the physics of sound intensity is frequency dependent either, but peoples perception of loudness most certainly is. As a rough guide, when I could play high and loud, I'd be pushing about a quarter litre of air per second into the instrument, so the PdV would be 10,000 * 0.00025 = 2.5 W. Your calculator gives 124 dB for 2.5 W/m2 so it seems to tie in. There will inevitably be some losses.
-
Acoustic Waves in Air with Variable Sonic Velocity
Depends on the frequencies and how close you are. Probably getting towards 120 dB at 1 metre.
-
Acoustic Waves in Air with Variable Sonic Velocity
A 10 kPa pressure amplitude swing around atmospheric should give a +/- 8 K swing around 300 K. That's a total variation of ~3% in sonic velocity. ie the peaks are travelling 10 m/s faster than the troughs. It's enough to seriously distort the waveform and raise questions about the usual sinusoid assumptions.
-
Acoustic Waves in Air with Variable Sonic Velocity
- Acoustic Waves in Air with Variable Sonic Velocity
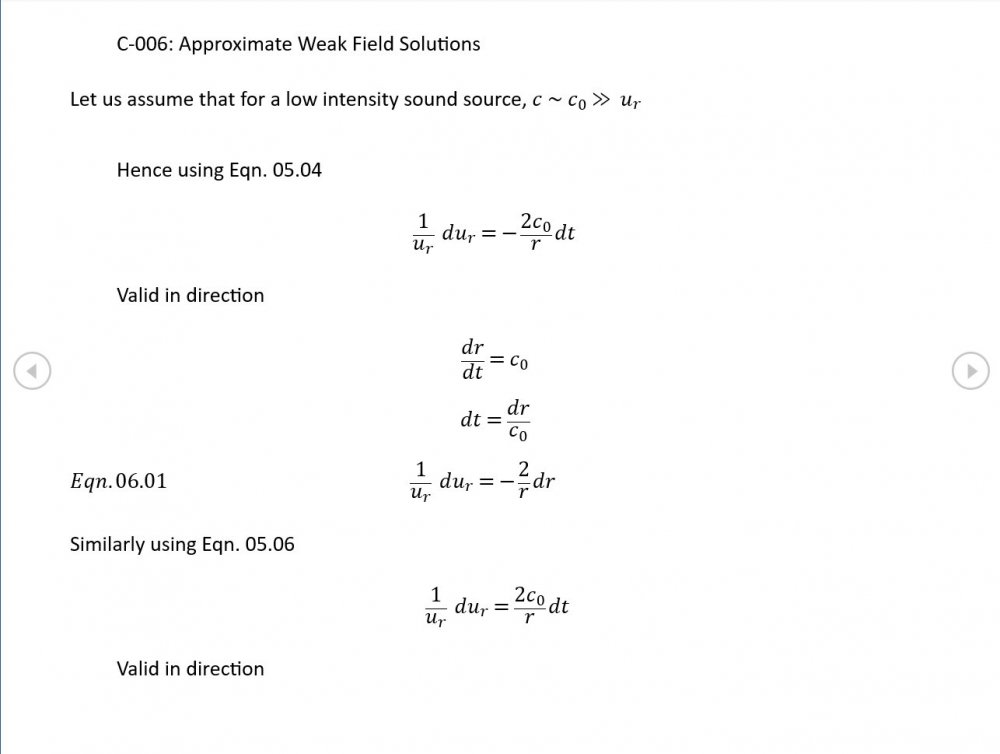
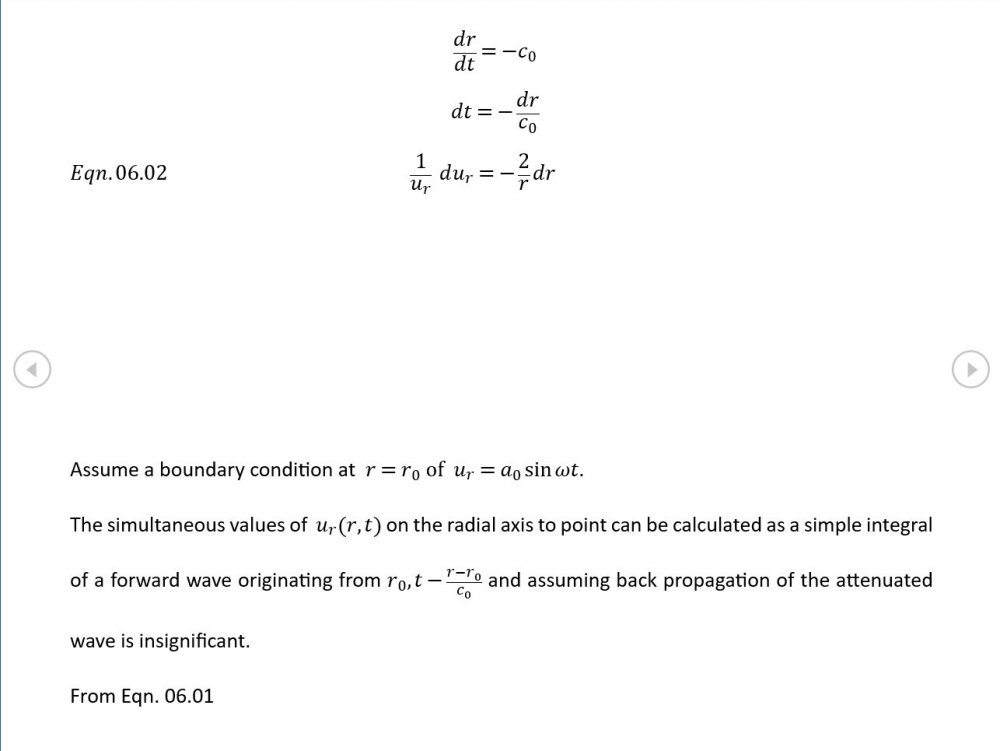
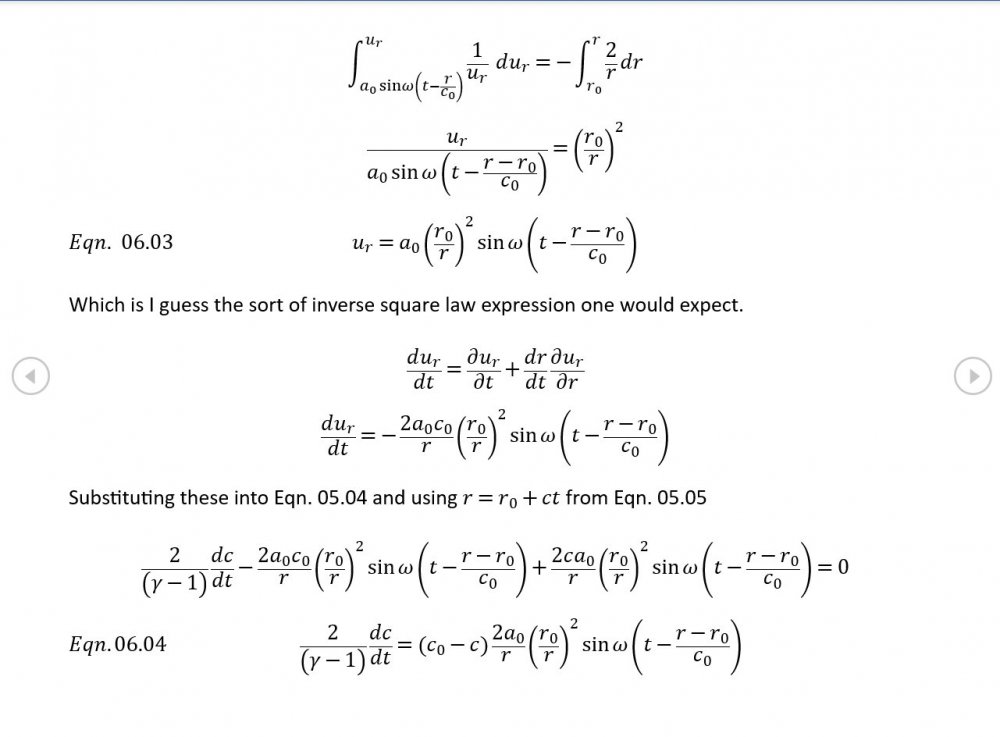
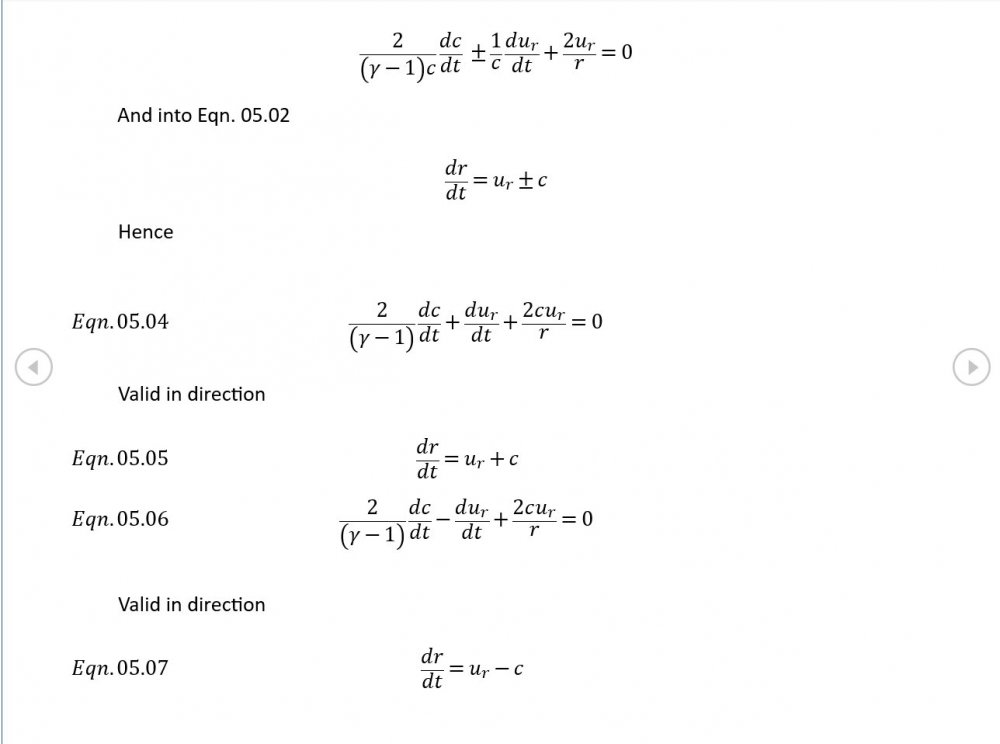
5.04 is integrated along 5.05, the line dr/dt = u + c, to give the forward wave. 5.06 is integrated along 5.07, the line dr/dt = u - c, for the return wave. Trumpets only exceptionally run above 10 kPa gauge and that's well within ideal gas range. PS You really DON'T want to hear acoustic waves with pressure amplitudes significantly higher than that.- Acoustic Waves in Air with Variable Sonic Velocity
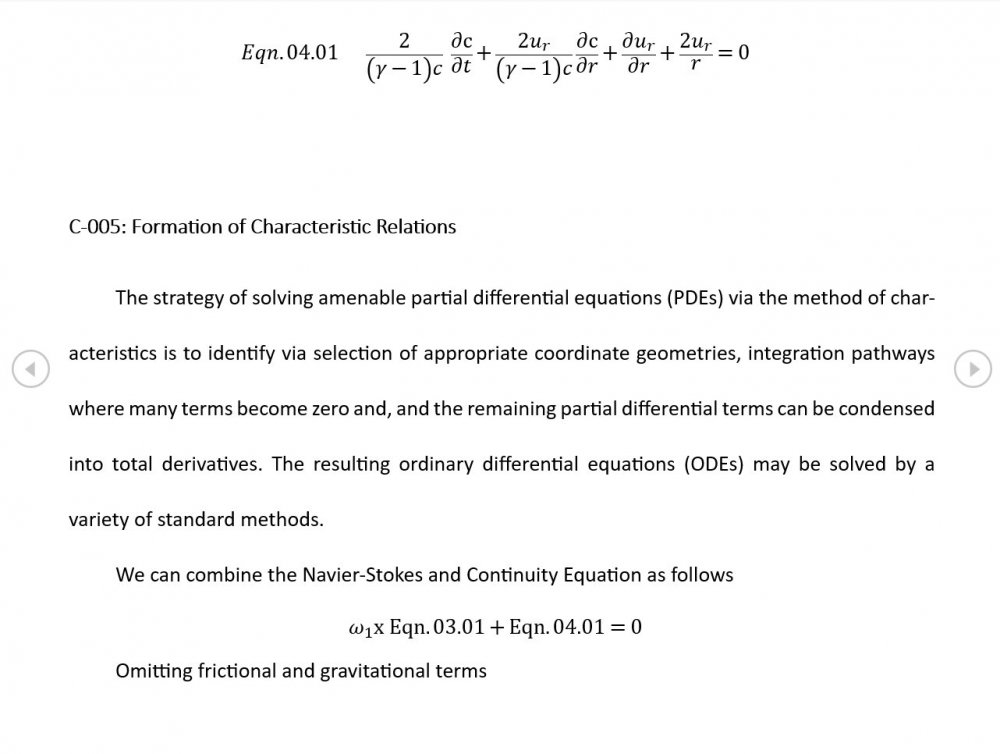
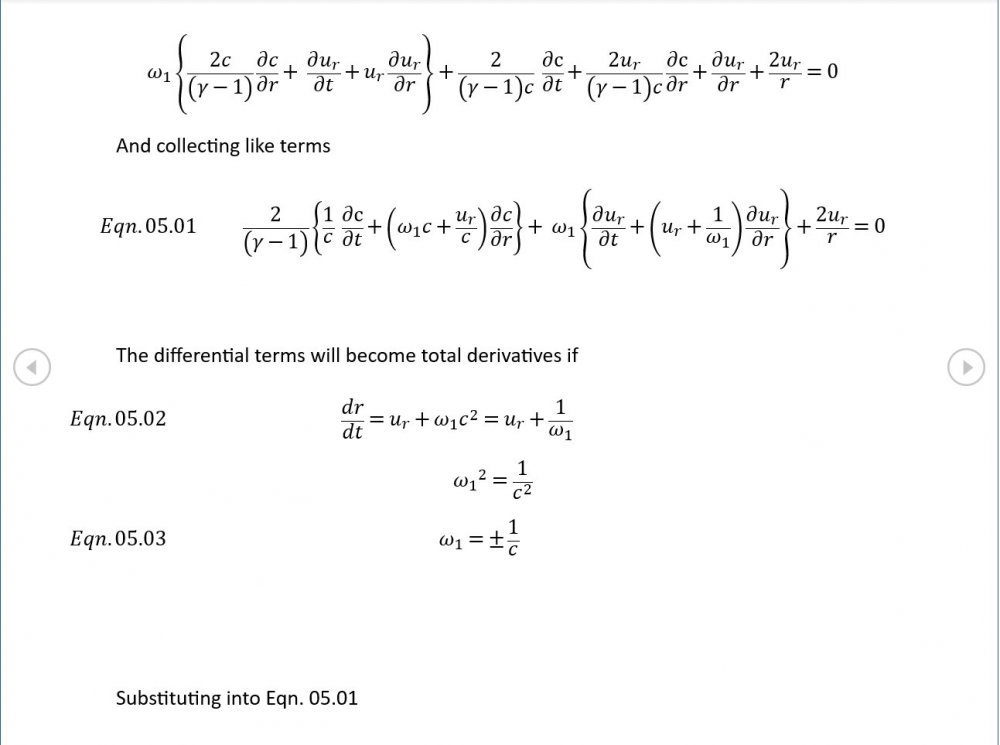
Just about every work I've read on air acoustics (I've read a lot) starts by assuming constant sonic velocity. I sort of understand the reasons for this, but it simply isn't accurate. The peak of the pressure wave is hotter and the trough cooler due to compression/expansion and this does affect sonic velocity. I believe it's significant enough to be a major factor in eg. developing the characteristic timbres of wind instruments which nobody seems to have got a proper theoretical handle on yet. I've made a start on developing a mathematical system modelling a spherical wave accommodating a variable sonic velocity and attached a brief summary. I'd be most grateful if someone would give it a quick once over to see if I've made any blunders along the way. The pair of simultaneous ODEs I've come up with are beyond my skills to solve analytically, but they're quite amenable to numerical integration. Any hints from the more mathematically gifted would also be much appreciated. Spherical Adiabatic Acoustics.pdf- dark matter question
So while the dark matter does experience some gravitational attraction to the galactic disc, it just ploughs straight through it and out the other side without losing momentum whereas normal matter will inevitably dissipate heat due to friction during transit, and ultimately after a few transits will join the disc. Have I got that right?- Apply Wind Code or CFD modelling?
I think the OP may fall more into the orbit of BS 5950 than BS 6399.- Making some ethanol... [only for when you are reaaaaally bored !]
To get methanol levels down to those of London Gin, you must use multiple stages of fractionation such as the Coffey Still (modern separation technology could effectively eliminate methanol from the product quite simply). To enforce those levels across the drinks industry would effectively ban the use of pot stills. A lot of people would get upset about that. - Acoustic Waves in Air with Variable Sonic Velocity
Important Information
We have placed cookies on your device to help make this website better. You can adjust your cookie settings, otherwise we'll assume you're okay to continue.