-
Posts
792 -
Joined
-
Last visited
-
Days Won
1
Content Type
Profiles
Forums
Events
Everything posted by Butch
-
You are stating exceptions, I was stating general rules... Manganese is a poor conductor because it has many valence electrons(as I stated) "Metals have few valence electrons, which allows for metallic bonding and better than average conductivity..." Indium tin oxide (ITO) is a ternary composition of indium, tin and oxygen in varying proportions. Depending on the oxygen content, it can either be described as a ceramic or alloy. as an alloy it is a very good conductor due to few valence electrons and the relatively weak bond of those electrons to the structure. as a ceramic it is an insulator. neither of these however is a metal... It is an oxygenated compound.
-
Chadwick noted that a free neutron was extremely unstable, if this is so, should we not immediately shut down Fermi? As we will soon be running out of neutrons.
-
.thumb.jpg.cc169fb67f9c969aeace623286b9b5e6.jpg)
If an electron falls through the nucleus of an atom...
Butch replied to Butch's topic in Speculations
More simply put, if you add the vector of the sister electrons the average energy would always equal that of the energy of a single qm electron at an energy of it's greatest probability... That electron however would have a probability curve that would never allow it to be in the nucleus charge radius of the atom. This is most easily understood in the deuterium atom. 2 sister atoms one at its highest probability and it's lowest energy state, the other in the center of the nucleus charge radius, at its highest energy state with a vector 180 degrees opposite the valent sister and with a much greater magnitude such that the addition of the vectors would produce an average energy equal to that of a single qm electron at its highest probability. How is an electron created? How is a proton created? How is a neutron created? Since the valent electron has energy the addition of the vectors of the sister electrons would produce a resultant that would always place the singular electron outside of the nucleus charge radius. That is to say the combined vectors would always produce a resultant having less energy than the sister electron inside the nucleus charge radius. -
.thumb.jpg.cc169fb67f9c969aeace623286b9b5e6.jpg)
If an electron falls through the nucleus of an atom...
Butch replied to Butch's topic in Speculations
The sum of the vectors of all of the sister atoms would agree with qm. They are not bound together in the atom, they are strongly interacting, each sister atom takes it place eventually as the valent electron, only when the atom decays do the electron and proton seem to be bound as they are outside the nucleus charge radius, at which time they quickly dissociate. -
.thumb.jpg.cc169fb67f9c969aeace623286b9b5e6.jpg)
If an electron falls through the nucleus of an atom...
Butch replied to Butch's topic in Speculations
The instability of a free neutron (Chadwick). In the tritium atom, the interaction of 2 electrons with the nucleus causes a greater expansion (or perhaps I should say oscillation) of the nucleus charge radius, causing greater instability of the atom, indeed deuterium is less stable than hydrogen1. Hydrogen one is ultimately stable. The thing that really draws me to this model is that if we add another proton electron pair to the tritium atom we either get a very unstable isotope of hydrogen or a helium4 atom! Note that in my model the neutron is actually an electron inside the charge radius of the nucleus, hence if the neutron decayed it would simply mean that a proton electron pair (or pairs) would be ejected from the nucleus. The pair would be tightly bound however the electron would be high energy, hence they would immediately dissociate, observed as the decay of a free neutron. -
.thumb.jpg.cc169fb67f9c969aeace623286b9b5e6.jpg)
If an electron falls through the nucleus of an atom...
Butch replied to Butch's topic in Speculations
In my model it has 3, 2 of which are interacting strongly with the nucleus. -
.thumb.jpg.cc169fb67f9c969aeace623286b9b5e6.jpg)
If an electron falls through the nucleus of an atom...
Butch replied to Butch's topic in Speculations
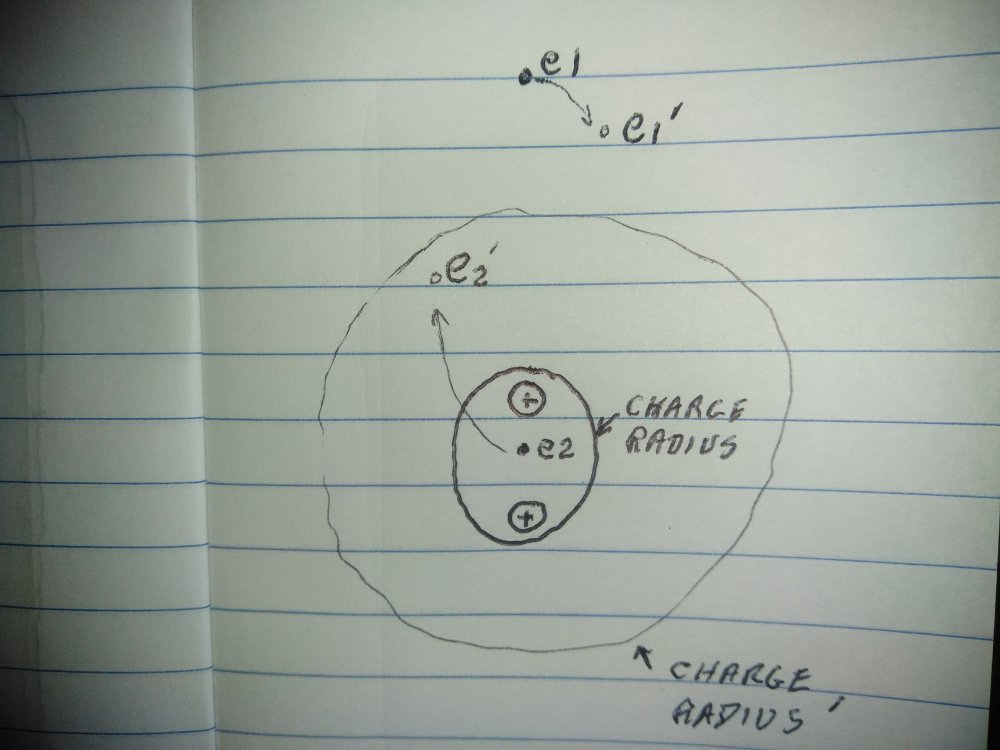
Ok, scratch all that... I should be thinking of particles as magnetic dipoles and centers of mass, correct? Still, the radius of the atom... The entity that I have referred to as the nucleus area would be the charge radius of the nucleus, this fits with my atom. Certainly not to scale. e1 and e2 are sister electrons when e1 is at its highest probability position e2 is near the center of the charge radius of the nucleus. e1 is the valent electron e2 is contributing to the charge and mass of the nucleus, the charge of the nucleus is +1 and the mass of the nucleus is equal to the rest mass of the 2 protons + the rest mass of e2 + the apparent mass due to the velocity of e2. The apparent mass(and thus the velocity) of e2 should be the difference in rest mass of a proton and a neutron. As e1 begins to fall towards the nucleus e2 which has greater velocity moves away from the nucleus, thus the charge radius of the nucleus increases. When e2 "passes" e1 the charge radius of the nucleus begins to decrease. In this atom: Valence electrons = 1 Charge of the nucleus = +1 Mass of the nucleus = 3.3475517 e-27 kilograms. -
Metals have few valence electrons, which allows for metallic bonding and better than average conductivity... Metal oxides have their valence band filled and are very good insulators, metal oxides are in fact ceramics (like glass). They do however make great magnets!
-
.thumb.jpg.cc169fb67f9c969aeace623286b9b5e6.jpg)
If an electron falls through the nucleus of an atom...
Butch replied to Butch's topic in Speculations
Which is not in accordance with experiment, so it is wrong. There is a shift because of the different mass of the nucleus, but the values do not vary. I stand corrected, the energy would have to vary... However the sum of the energies would have to equal the energy state of the atom. I have some of the math for my model, indeed a good chunk of it and the rest is not difficult thanks to qm. The shift is somewhat more intriguing than you might think. Checking my thinking, is the diameter of deuterium different than that of hydrogen1? I suppose there are other explanations, the space between protons could vary or (unlikely) the density of the proton could change along with it's diameter. Any of these ring true? I did not say their could only be 2 sisters, tritium has 3 sisters. I think that indirectly qm solves this for us, take all those vectors together and we have h/2pi or -h/2pi, with qm it is just so much simpler. Checking my understanding, when an electron shifts to a higher energy level it moves to a lower "shell" or a higher shell(still trying to pull my head out of my clASSical). -
.thumb.jpg.cc169fb67f9c969aeace623286b9b5e6.jpg)
If an electron falls through the nucleus of an atom...
Butch replied to Butch's topic in Speculations
And the component vectors of this quantity could vary? In the atomic model I will be attempting to describe in terms of qm "sister" electrons would have synchrony in that at any instant the sum of the angular momentum of the electrons would equal the energy state of the atom, while the angular momentum of the individual electrons would vary the sum would not. In hydrogen1 the electrons energy would not vary, however hydrogen2 is a different story. -
.thumb.jpg.cc169fb67f9c969aeace623286b9b5e6.jpg)
If an electron falls through the nucleus of an atom...
Butch replied to Butch's topic in Speculations
Yes, I restated my previous post, is it correct? -
.thumb.jpg.cc169fb67f9c969aeace623286b9b5e6.jpg)
If an electron falls through the nucleus of an atom...
Butch replied to Butch's topic in Speculations
As far as qm is concerned, no it does not vary... However an electron can have many states, qm derives the highest probability state... So would it not be possible that the amplitude of the angular momentum vector could vary? -
.thumb.jpg.cc169fb67f9c969aeace623286b9b5e6.jpg)
If an electron falls through the nucleus of an atom...
Butch replied to Butch's topic in Speculations
Because the angular momentum is an average or perhaps a constant in qm, could we refer to it as a pseudo vector? That is as a vector set that cycles through 360 degrees and may or may not vary in amplitude? I see your point, I was still thinking paths in that the path length(in terms of time) would equal wavelength, sorry hard keeping my head in qm rather than classical. -
.thumb.jpg.cc169fb67f9c969aeace623286b9b5e6.jpg)
If an electron falls through the nucleus of an atom...
Butch replied to Butch's topic in Speculations
I'm not sure what that is supposed to mean. The ground state electron is most likely to be found at a distance from the nucleus equal to the Bohr radius, 1s atomic orbital (sorry for using that word again, I am transitioning to qm) If the single electron in the hydrogen atom is at ground state would it be incorrect to refer to the atom as being at ground state? Regardless, if the electron is at ground state would I be correct to say it has a wave function of 1? Would wave function relate to angular momenta akin to the relation of a circle and a sine wave? That is to say, angular momenta indicates a changing vector if the electron is to remain with the atom that vector must transit until it returns to its original state relative to the nucleus. -
.thumb.jpg.cc169fb67f9c969aeace623286b9b5e6.jpg)
If an electron falls through the nucleus of an atom...
Butch replied to Butch's topic in Speculations
Ok, so no matter the path of the electron, it must cycle true to it's wave function. Any excess energies are attributed to spin... Correct? A hydrogen1 atom at ground state would have a wave function of 1? Correct, but it would wobble and in more complex atoms that would have to be accounted for because of the periodically close proximity of the electrons to the nucleus. Ok, thanks to qm, the wobble does not matter and thanks to wave function the math is simpler. Thank you... Again! -
I have educated myself now to the point that I understand that matter and em are no different when it comes to wave function. Thanks all.
-
Yes, thank you, graphs and geometry... Although I did have some trouble conveying simple acceleration in a graph recently.
-
.thumb.jpg.cc169fb67f9c969aeace623286b9b5e6.jpg)
If an electron falls through the nucleus of an atom...
Butch replied to Butch's topic in Speculations
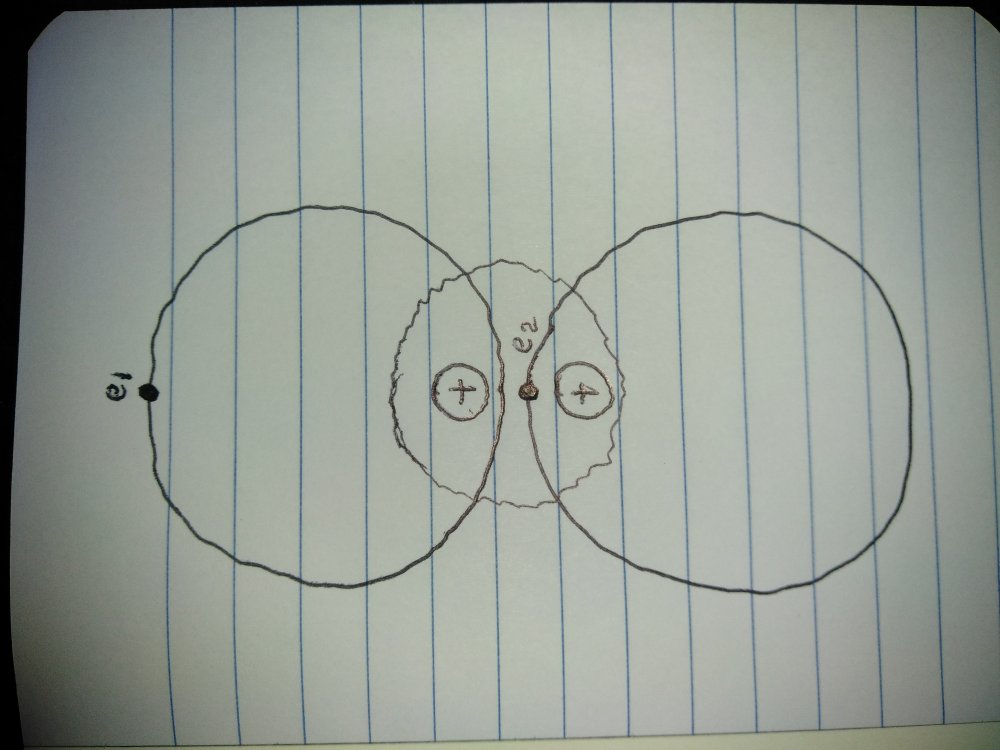
So... The wave function would be the same for both but the spin would be opposite and if the wave function for e1 was 2, the wave function for e2 would have to be 2 for this system to maintain balance. I take it that even though my electrons "fall" through the nucleus, the wave function really is about cyclical time, not altitude of orbit and even though my electrons accelerate and decelerate unless their average energies change their wave function remains the same? -
Well, I do have my head around wave function now... I think.
-
.thumb.jpg.cc169fb67f9c969aeace623286b9b5e6.jpg)
If an electron falls through the nucleus of an atom...
Butch replied to Butch's topic in Speculations
Trying to understand wave function, in this atom: How would I describe the relationship of e1 and e2 as wave functions? -
No, I equate it to a precessing orbit, am I even close to correct? I know I am expressing this in classical terms.
-
Is there evidence of the particle nature of light in laser interaction?
-
I am learning qm (self teaching) and I am keeping my head on a swivel so I don't miss anything. Such nature of matter would be the anti thesis of light quanta and I would like to see how it was eliminated as a possibility. Can you provide information or a link?
-
Thanks! More correctly angular momentum not accounted for by a flat orbit?
-
When an electron is said to have spin, what exactly is spin?