exchemist
Senior Members-
Posts
4500 -
Joined
-
Last visited
-
Days Won
72
Content Type
Profiles
Forums
Events
Everything posted by exchemist
-
This is not so much a question about chemistry as about the shelf life of specific manufactured items. I found this link, which may point you in the direction of an answer: https://electronics.stackexchange.com/questions/8794/do-electrolytic-capacitors-have-a-limited-shelf-life From this it rather looks as though trying to put into service a 30yr old component may not be a good idea.
-

Balancing a pools pH with Boron in the water
exchemist replied to NotYou's topic in Inorganic Chemistry
I don't know about a formula, but this link: https://www.borax.com/BoraxCorp/media/Borax-Main/Resources/Brochures/borates-swimming-pools.pdf seems to contain a lot of information about the use of borates in swimming pools. It seems to act as a buffer, enabling the chlorine to work in its best regime and also to soften the water by binding calcium. If it acts as a buffer, it may be that by using borates you can fully control the pH with them alone and can dispense with the hydrochloric acid entirely, but I stress I am learning all this as I go in response to your queries, so you might want to do some further checking. -
It is not strictly true that water is non-compressible. Liquids are about as compressible as solids, which is to say hardly at all by comparison with gases. But they still compress a bit. If you suddenly expose the water in a torpedo tube to 100atm, you exert that pressure on the water inside and thus on the walls of the tube as well. The water will compress a tiny bit and the walls of the tube will stretch and expand a tiny bit as well. (Because it is only a tiny bit, very little work is done, so there will be very little stored energy in the compressed and stretched materials.) Water hammer is a shockwave caused by abruptly blocking the path of a moving mass of water, thereby causing rapid change in momentum. This change of momentum requires a certain impulse (F x t) and because t is so small (because it happens fast), F has to be great. So that means water hammer creates large forces and hence pressures - a pressure wave. When you open a torpedo tube that is full of water, you do not have this, because the water on both sides of the opening is static and no change of momentum occurs. So it won't cause water hammer, just a bit of stretching of the walls of the tube. As for the equalising valve, I suspect that will be because when you flood a torpedo tube in practice you most certainly do have trapped air, which will compress to 1% of its volume, storing a lot of energy and causing water to flood in as it is compressed - with momentum. So there can be large forces and energies created in that scenario, which you do not want for safety reasons. So you flood it progressively rather than instantaneously. At least, that would be my best guess as to what is going on.
-

What is the size and shape of single optical photon?
exchemist replied to Duda Jarek's topic in Physics
Yes I suspect you touch on something important here. I'm halfway through Carlo Rovelli's book "Helgoland" at the moment. He points out that Heisenberg's approach to QM was based on deliberately restricting the model to accounting for the behaviour of systems in interactions - and not making any assumptions about what goes on in between. It is the classical mindset that assumes something goes on in between that can be defined and tracked. QM gives up that assumption. Or so I am led to understand. I feel it is not a coincidence that @Duda Jarek's posts and links continually refer to classical or semi-classical models. I suspect this is all an exercise in semi-classical modelling and should not be taken seriously as the way nature really behaves. -

What is the size and shape of single optical photon?
exchemist replied to Duda Jarek's topic in Physics
I'm still struggling to see what the "dimensions" of a photon, or even expectation values for a set of dimensions for an ensemble of them, can mean. According to my understanding, QM only describes how quantum objects are expected to interact (usually expressed in terms of probability distributions) and is deliberately silent on what they "do" in between. Do any of these authors suggest that the "shape" or "dimensions" of a photon predict how it will interact with other QM objects? If not, then it seems to me to be just building castles in the air. -

What is the size and shape of single optical photon?
exchemist replied to Duda Jarek's topic in Physics
My limited, chemist's understanding of QM is that you can't really speak of an "objective" EM field configuration for a single photon. If you could, it seems to me it would be a classical object rather than a QM one. But I think we probably now need a real physicist's input. -

What is the size and shape of single optical photon?
exchemist replied to Duda Jarek's topic in Physics
I'm sure you can say something about the distribution of probability of detecting the energy. -
Ah, so the salt is used to generate chlorine by electrolysis - which I can see makes sense, if you don't mind swimming in salt water and the associated potential for corrosion, I suppose. I didn't know that. Thanks.
-

What is the size and shape of single optical photon?
exchemist replied to Duda Jarek's topic in Physics
To be honest I have trouble seeing how asking what the "shape" of a photon is can possibly be a question with any meaning. One could only define a "shape" if one could find a way to interact with it in a way that did not disturb it, which does not seem possible to me. It also seems to me the uncertainty principle would suggest its extent in space would depend on the degree to which its momentum was defined. This seems to be merely an academic exercise in exploring, for fun, the ramifications of the Bohr model - which was abandoned as a model in the 1920s, due to its obvious inadequacies. I also note the paper is dated 2018, a decade after this George Hunter bloke, whoever he may have been, died. But I'm not a physicist. There are others that can comment more authoritatively, I'm sure. -
Those stains look like iron salts to me. You'll have to explain to me why you associate them with salt, as I'm a Brit and we don't have many outdoor swimming pools here. Do you use salt to treat the water in some way? Could it have iron as a contaminant, like the rock salt we put on the road in winter, which always looks a bit pink or brown? Chemically, I would expect ascorbic acid to form a "chelate" with iron Fe³⁺ ions, which is a sort of cage molecule enclosing it. This could serve to dissolve the iron salts off the sides of the pool, if that it what it is.
-

Dissolving Nanoparticles (TiO2) in epoxy resign
exchemist replied to darius222's topic in Applied Chemistry
This looks like a question for someone with experience of the paint or allied industries. I can't answer this myself but I see from this: https://www.crayvalley.com/docs/technical-paper/dispersing-titanium-dioxide-with-sma-resins-(1).pdf that TiO2 is sometimes indeed given a surface coating, in this case involving Al2O3 and something organic. It may be that either Cray Valley or Millenium Inorganic Chemical could advise you further if you contact them. -
It certainly looks as if it has pink feldspar, black mica and white quartz, so could be granite. It doesn't look porphyritic to me, in that there is no glassy or microcrystalline matrix. But I'm no mineralogist. It's rather a pretty rock, actually.
-

Electric Vehicle Batteries - A 10 Year Time-Bomb ?
exchemist replied to studiot's topic in Science News
This looks like a case for early legislation, mandating that manufacturers take back expired batteries for recycling. From the article, the technology to do it does exist, so there is no need for it to be like nuclear waste that has to be just put in a hole in the ground. But it will be costly and nobody commercial will do it just out of the goodness of their hearts, so legislation must be the way to go, I think. -
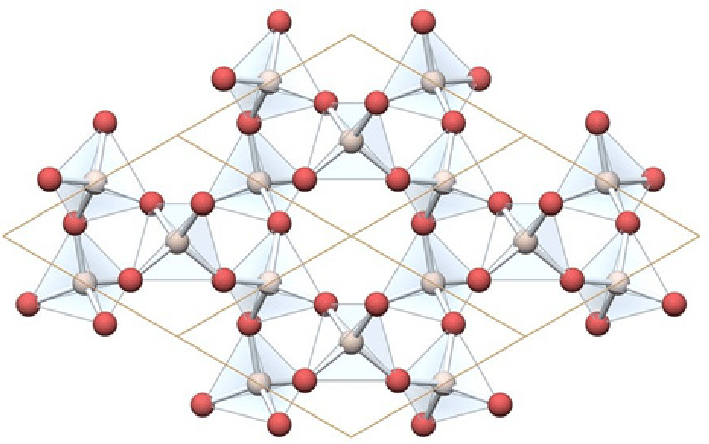
You're right, it's the empirical formula. Silica (quartz) is a covalent giant structure. As such, there is no molecular formula, since there are no discrete molecules in the structure. You could almost say that an entire crystal is in effect a single "molecule! So for giant structures, the empirical formula is what we use. Here is a picture, in which you can see the SiO4 tetrahedra sharing the O atoms at their vertices with their neighbours:
-
I don't know anything about this myself, but I did find this article by a wood panel manufacturer, who talks about the effect of pH of the wood on curing of adhesives:https://www.wbpionline.com/features/ph-and-why-you-need-to-know-it/ If you are interested in veneers, it occurs to me that you too may be concerned with adhesives.
-

Suggestion for teaching force components on ramps
exchemist replied to ScienceNostalgia101's topic in Physics
I don't find this especially simple to grasp, actually. Though maybe it is if you stand in front of a class explaining it as you go. What I always used to do is consider what would happen if the angle went to zero. The Cos component is the one that goes to full value and the Sin component is the one that goes to zero. -
Is this a trick question? I'd have thought the answer is "easily" - if you mean faster relative to some external observer.
-

Does adding acid to a pool lower its hardness?
exchemist replied to NotYou's topic in Inorganic Chemistry
I don't think it will. "Hardness", so far as I understand it, relates to the concentration of Ca²⁺ and Mg²⁺ ions in the water. This will not be affected by adding HCl. What you may do is reduce the amount of carbonate and bicarbonate in the water by lowering the pH and causing some of these to be converted to CO2, which is lost to the atmosphere. But you are introducing chloride Cl⁻ ions instead, so you are effectively replacing dissolved CaCO3 and Ca(HCO3)2 by CaCl2 - which I believe still counts as "hardness" according to most definitions. However, reducing the amount of carbonates and bicarbonates will reduce the tendency of these to precipitate out as scale deposits, so it may look like a reduction in hardness in practice. As least, I think that is how it works. Others more knowledgeable may correct me. -
Haha, that's why I included the qualifier "mainly".😉 But as it happens, speaking as someone who had a Catholic upbringing, even if I have been semi-detached for many decades now, there is actually no testable claim made for transubstantiation either. See "essence" vs. "accidents", for the traditional (rather itchy-beard, to my mind) way of getting round this. I would treat the major established Western Christian denominations, including Catholicism, as among the "more reasonable manifestations", along with many branches of Judaism and educated Islam. I know less about other religions but would expect to be able include some of them too. All of these are mainly a guide for living one's life and do not try to offer an alternative narrative to science about the way the physical world works.
-
Shell: Anglo-Dutch, but with a large and semi-autonomous US arm. But I have in mind not only Shell itself but the companies we used to do business with, either as suppliers to us or as our customers, or as manufacturers whose machines used our products: I was exposed to all three in the course of my career, many of them American companies. There were some, usually smaller, who you could perhaps characterise as cynical and driven only by short term profit, but most of the larger ones took a much more nuanced approach to their business.
-
Speaking as someone who worked for an oil major for over thirty years, including a short spell in the US, I find this unduly cynical - or a bit naive. My experience is that while major corporations certainly are driven by the bottom line, as they should be for the sake of their shareholders, it is not that simple. A major issue for companies like mine was the long term reputation of the brand, which was seen as essential to secure a "license to operate" from society - and thus protect long term profitability. There was also considerable pride in the standards of the company, in such matters as product quality, engineering and above all safety. (The safety culture was extremely tough: people could be - and were - sacked for not switching off their mobile phones when driving, for example. Being at work in one of the oil refineries was, famously, considerably safer than being at home!) So it is a bit glib and superficial to claim that the profit motive drives towards unsafe products and working conditions. It may in some companies, but not in well-run ones. On taxation you are right, of course. No company will pay more tax than the law requires and multinationals do jump around to find the lowest tax rates. But this is driven by governments competing to offer the lowest rates, in order to attract business. This is an argument in favour of countries agreeing to stop this practice and start to harmonise corporate tax rates - as I gather Biden is now proposing, in a modest way.
-
This seems to be incorrect. So far as I am aware, antimony does not expand on freezing. You may be thinking of bismuth. And silicon and gallium also do this - along with water. Antimony was indeed used in typesetting, but alloyed with lead, not on its own, in order to reduce the degree of shrinkage on cooling, as well as to make a harder alloy to resist damage in the printing process. It did not cause expansion.
-
Glad it helped. By the way I see I made a typo in the formula for silica, which should be SiO2, not SiO4. (Although the units are SiO4 tetrahedra, by the time they share all their "O" vertices with neighbouring ones, the overall ratio of O:Si becomes 2:1.)
-
The first point is that all these elements are present in the form of compounds, in which their atoms are chemically bound to other atoms, whether by ionic or covalent bonding. The boiling point of oxygen (O2) is therefore not relevant, since free molecular oxygen is not what is being referred to. As @studiot says, oxygen is mostly present in magma as various kinds of silicate. The chemistry of silicates is very complex, but is all based around variants of the tetrahedral SiO4 unit, sometimes free but more often joined to others by shared vertices, to form chains, sheets or 3D arrays. These units are covalently bonded but tend to have a net -ve charge, thereby forming a family of silicate anions (SiO4⁴⁻, Si2O7⁶⁻, and so on) that can complement the metals you list, since they will be present in the form of cations. (You may be familiar with other complex anions such as carbonate CaCO3²⁻, sulphate SO4²⁻ etc, which also have covalent bonding internally but a net -ve charge overall. Silicates are like that.) However it is worth noting that pure silica itself, SiO4, in which SiO4 units form a 3D array, has no ions. When this melts, it requires some of the covalent bonds to break, temporarily, and reform, allowing the units to slide past one another. You get silica in most magmas, along with various silicates.