-
Posts
56 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by nancy9494
-
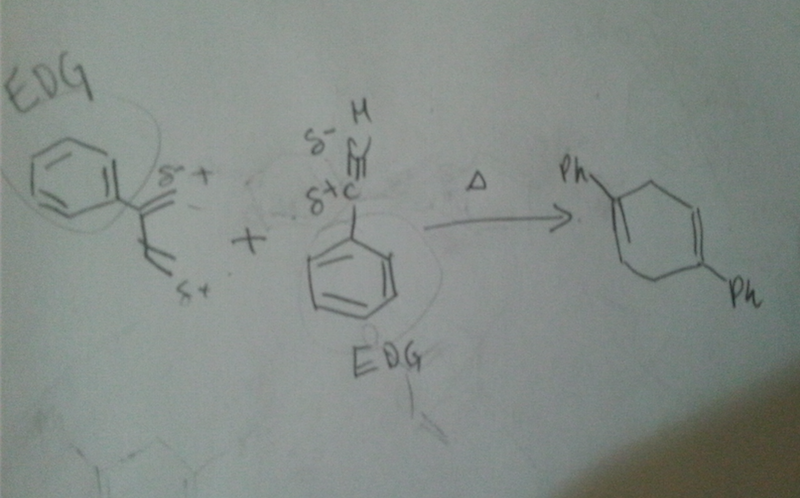
Hello friends I dont understand how the end product is the answer. I have labeled the partial charges. Did I do them wrong? My reasoning is that the ph are EDG. thank you in advance
-
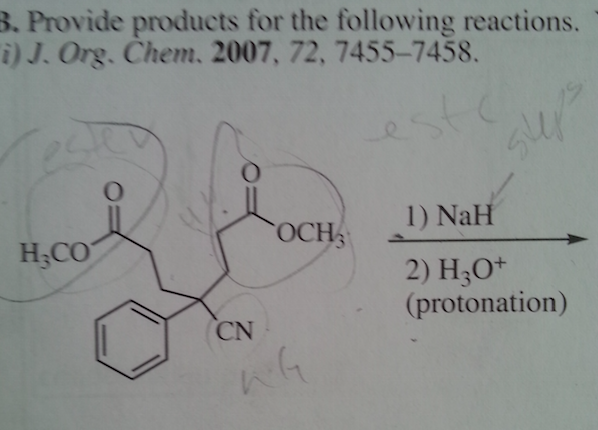
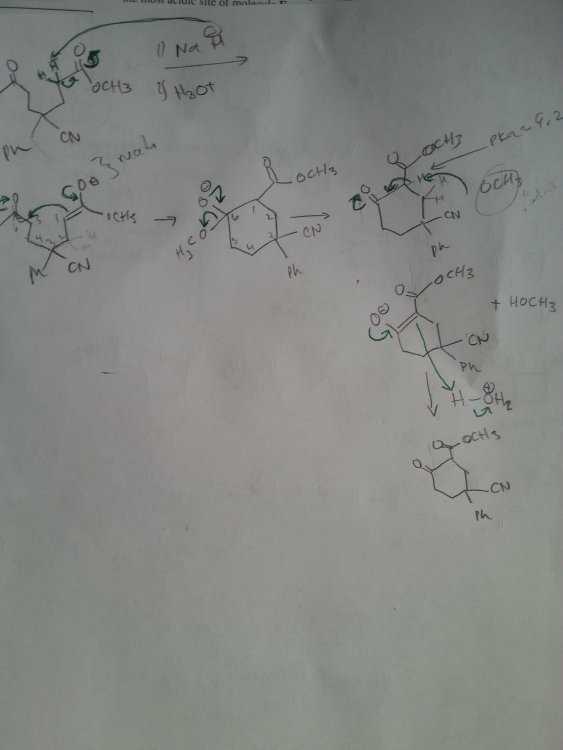
There are two ester groups so I think it's a Dieckmann condensation, but that CN is making me nervous. I think the esters make the alpha more acidic than the CN does that is why I deprotinated the H by the ester. Any help is appreciated thanks
-
very helpful thank you!
-
I've looked at some school requirements and all they say is 2 years of undergrad lab work. Some-kind of experience is better than no experience I guess. thanks
-
Hey, I'm majoring in microbio and I want to pursue a PhD/MD. Does it matter what type of research I do as an undergrad? I am trying to get into a microbio lab but the spots are limited, but there are some spots open in some biomedical labs. Would it look weird if I got a position there?
-
ahh yes thank you
-
the OH
-
The water because it is under acidic conditions?
-
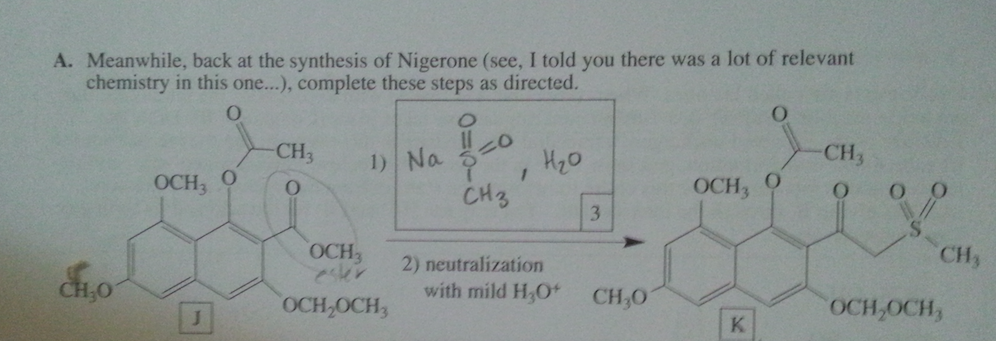
Hello, I don't understand where the H3O+ comes into play, so I could have missed the mark by using the base as acid base chem instead of using it as a acyl transfer.
-
So i think this is a hydrolysis of an ester, but I'm not sure. If it is, do I need to add water as a solvent? Most of the examples I have seen with an ester under basic conditions is that there is H2O written alongside the anionic base. Thanks in advance
-
I just don't understand why it can't chill in 3rd form. I can memorize the mechanism but I'd prefer to know.
-
better late than never? Why does the OH leave in the 4th step? why does this need to happen? thanks in advance
-
awesome thanks!
-
Hey so my professor did this example in class and drew one of the products, but he didn't mention the leaving group product that results. I have drawn the product I think that would result from the leaving group below. I don't know if it's correct. thanks in advance
-
out here saving lives! thanks again
-
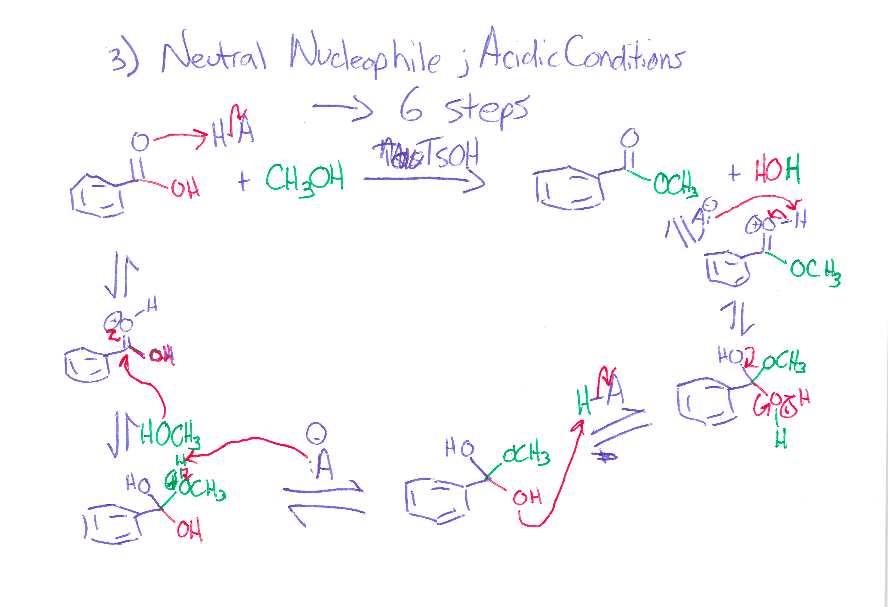
Never mind about this one. The professor went over it and the second step H3O+ is missing. The first one OH deprotinated by the CH3 then protonated by the the H3O+. Excess same as first but the another CH3 attacks the ketone and that is later protonated by H3O+ [The added CH3 is on a wedge]
-
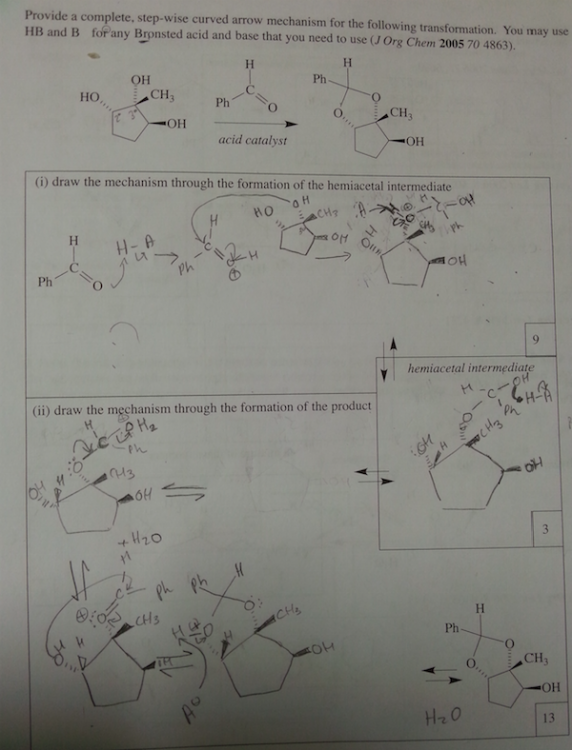
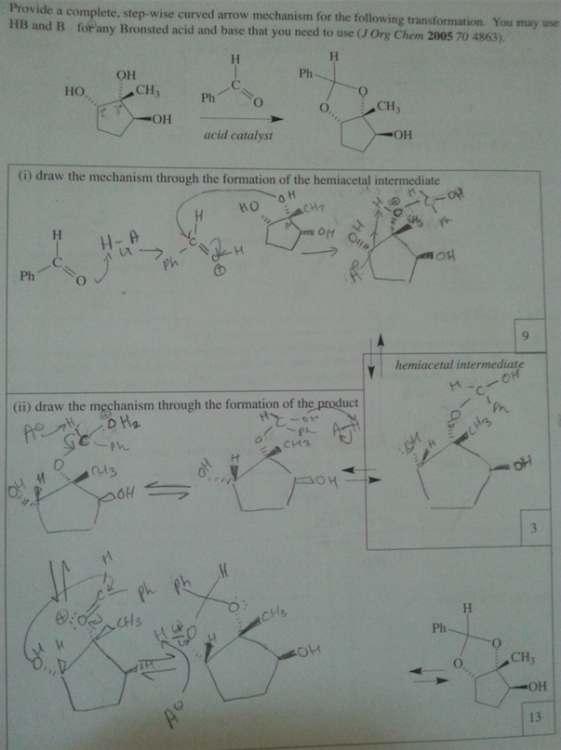
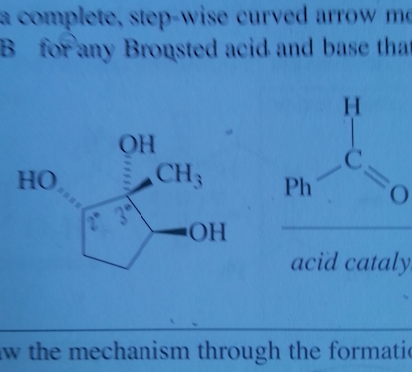
What is a supporting information document? Is that in the journal? We are not allowed to use references for the exam. I think they just want us to use our tool bag of Orgo 2 knowhow. It is supposed to be doable without it. sigh. I asked my GSI (graduate student instructor) and she said the first step is deprotination of the OH group. So I'm guessing if there's excess the C anion will deprotinate the OH and attack the ketone. The formal charges of the O's make me uncomfortable.
-
part ii) If the OH group is protonated with an acid, it can be kicked off by the nucleophilic oxygen when it forms a double bond with the C. Would it react with the OH that are on dashes faster because they are closer together and most of this process is intramolecular? Thank you so much for your help. I really appreciate it.
-
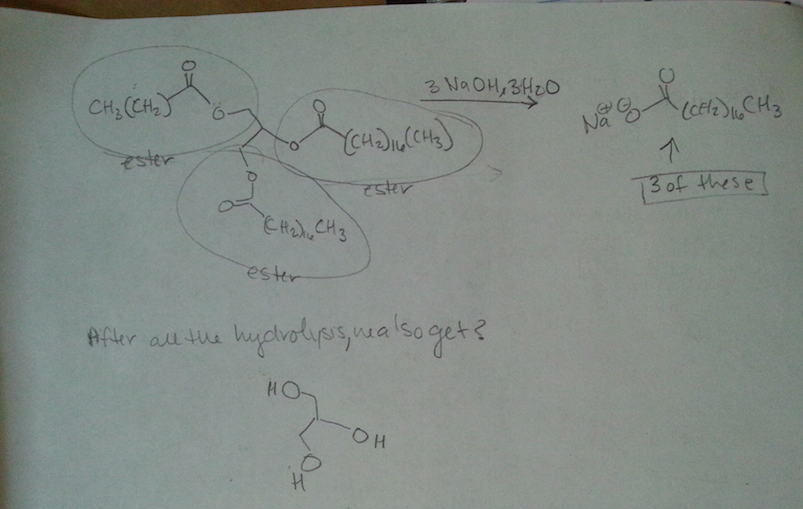
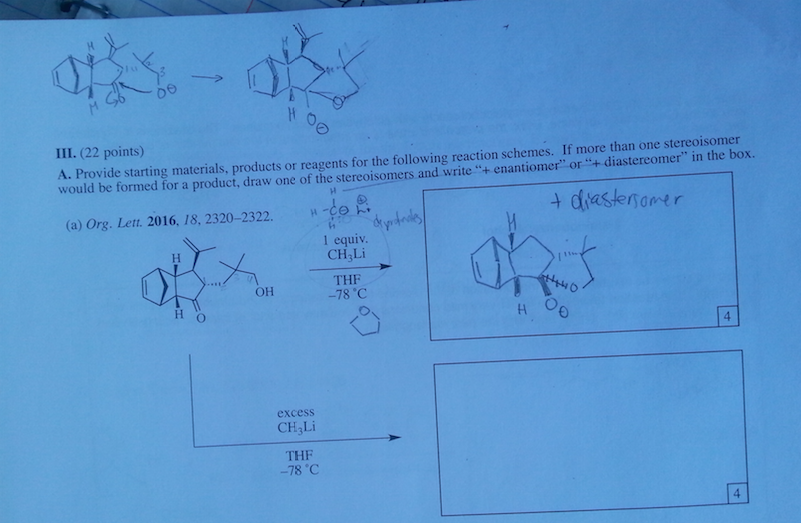
There are 3 OH and two of them are secondary so I think that's supposed to be a clue? I mean the end product will be the same, but they love taking points off.
-
Question: which OH is more likely to attack the aldehyde? I think it would be the one the tertiary C because the Positive charge that is created of the O will be stabilized through hyper conjugation.
-
The strong CH3 Nuc would deprotinate the OH rather than react with the ketone? If so is there a lewis acid source to protinate the O? Could I also get a hint on how the strong C [excess] would impact the compound?
-
as always thanks again!
-
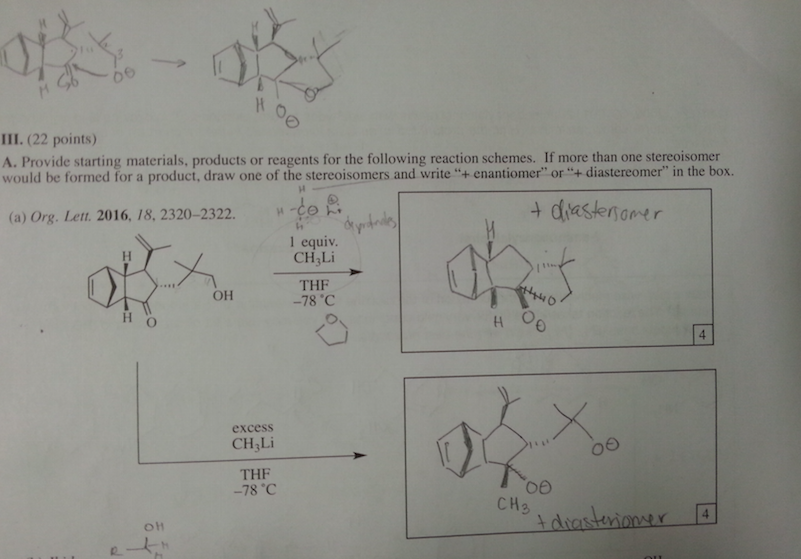
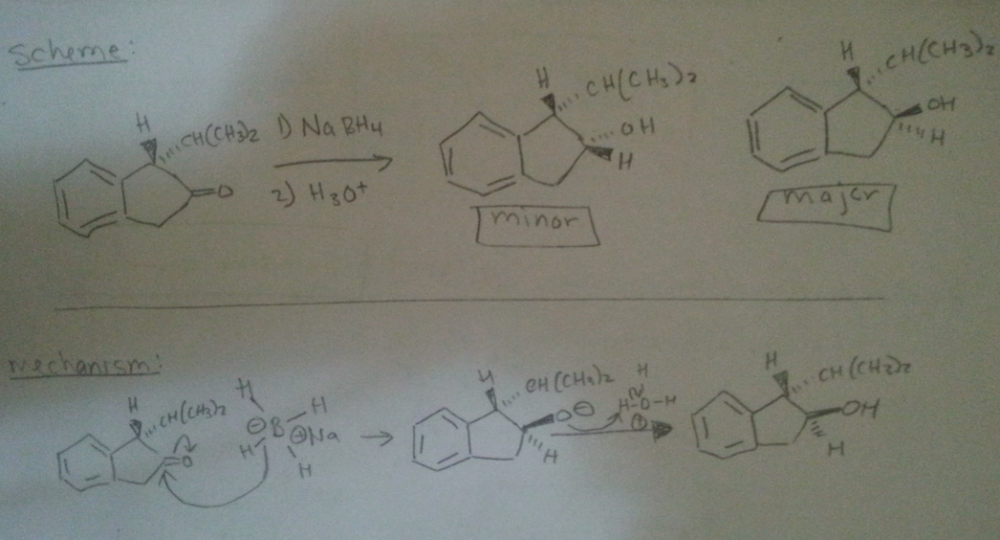
I'm confused. Shouldn't the larger substituents be opposite to each other? So the OH has to be oriented away from the CH(CH3)2. Did I label them incorrectly then? If so, is it because the strong nucleophile of Hydride anion is irreversible so the path of least resistance [front side attack] will determine the major product formed?
-
hello, I wanted to know if i labeled the possible products correctly [i.e. minor vs. major]. reasoning: Although there is less steric hinderance for the Hydride ion through front (because of sterics of the CH(CH3)2 group on the dash) that won't be deciding factor for the amount of major product formed. The back side attack will form the major product because the larger OH and CH(CH3)2 groups are anti to each other. Is this correct? thank you in advance