Rachel Maddiee
Senior Members-
Posts
108 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by Rachel Maddiee
-
sorry, I counted wrong.. Would I add them together? 2+4+ 8 I did 3 bond pairs=6 elections and 4 lone pairs=8 elections.. total 14 elections Oxygen has one bond pair and 3 lonepairs. Carbon has thrree bonds and a lone pair.
-
this is really confusing me. This is what I’m up to Incorrect structure: 8 bonding electrons and 2 lone pairs Number of electrons = 6 + 8 = 14 total electrons Correct structure; CH2O There are 4 valence electrons in carbon, 1 each in hydrogen and 6 in oxygen, so there are 12 electrons total. In the structure carbon has one lone pair and oxygen has three lone pairs which is incorrect.
-
So do I need to include that in my explanation?
-
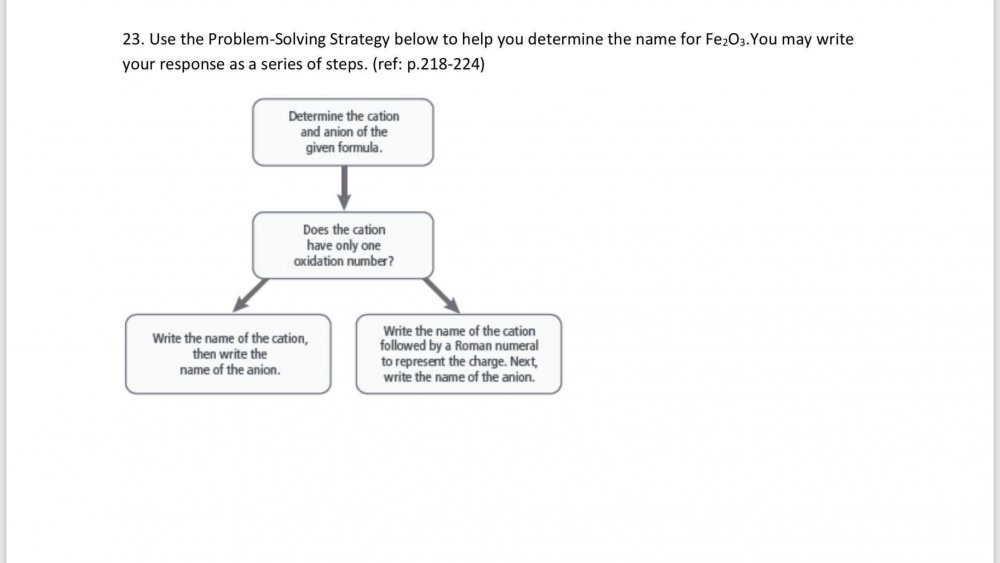
Does this do a good job of naming the compound and explaining how I determined it? Step 1 Cation: Fe+3 Anion: O-2 Step 2 Iron has two oxidation numbers Fe+2 and Fe+3 The element iron (a transition metal) has an electron configuration of [Ar] 3d6 4s2 with atomic number 26. It has two electrons in its 4s orbital and 6 electrons in its 4d orbital. When iron loses the two 4s electrons, it attains a valency of +2. It can lose one of the paired electrons from the 3d subshell (which provides a more stable configuration), and it attains a valency of +3. Step 3 Fe2O3 = iron(III) oxide
-
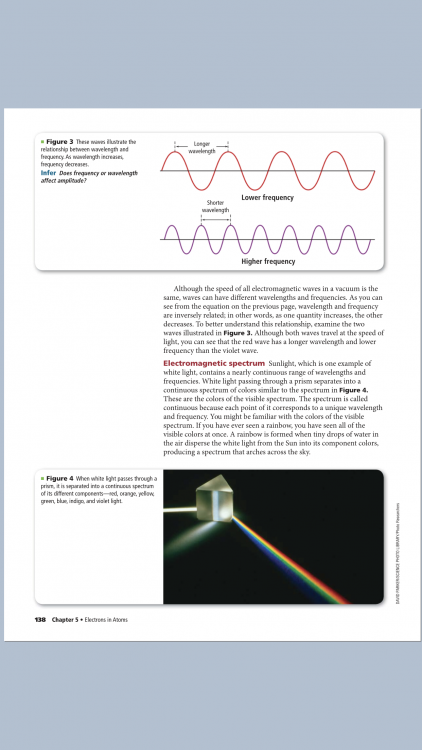
The amount of energy carried by a wave is related to the amplitude of the wave. A high energy wave is characterized by a high amplitude; a low energy wave is characterized by a low amplitude. ... But it does not ask about the relationship between amplitude and energy? What exactly did I miss in my explanation?
-
Wavelength and frequency are inversely related. I don’t really see anything about the amplitude in that information? The speed of light is equal to the product of the wavelength and frequency
-
Would there be 14 total valence electrons? the numbers don’t match
-
Sorry, typo.
-
-
You said that you only count the valence electrons once. How do I do that? Number of electrons = 6 + 8 = 14 CH2O There are 4 valence electrons in carbon, 1 each in hydrogen and 6 in oxygen, so there are 12 electrons total.
-
and counting the valence electrons will help me determine which rule is incorrect? I’m trying but I don’t understand how you calculated to find the valence electrons
-
how do I determine which rule it does not follow?
-
I know that Carbon has 4 valence electrons and oxygen has 6.. I’m not sure if I’m counting the 6 from the lone pair?
-
so Since the oxygen and carbon end up with full shells, do they hold to the rule?
-
I’ve been having trouble with the description for this. So carbon has 8 electrons and oxygen has 6?
-
Note that these are valence bonds, not ionic bonds, so the valence electrons are "shared" between the bonded atoms rather than being gained by one atom and lost from another. So would it be an incomplete octet? oxygen has four non-bonding lone pairs remaining in its outer shell, so it will have two lone pairs.
-
-
Energy increases as the electromagnetic wavelength decreases and the frequency increases. The energy of a wave is directly proportional to its frequency. A wave will transmit more energy per second if it has a higher frequency. Shorter wavelengths are more energetic than longer wavelengths. Is this correct?
-
Naming Oxyacids (oxyacids produce the H+ cation) The first step in writing a name for a formula is to decide which type of compound it represents. Determine the anion (the name of the acid is based on the anion attached to the hydrogen) The acid name comes from the root name of the oxyanion name or the central element of the oxyanion. Oxyanions are named according to the following rules: If the group ends in the suffix -ate (with more oxygen atoms bonded), it changes to -ic If the group ends in the suffix -ite(with less less oxygen atoms bonded), it changes to -ious Is this step process correct?
-

Chemistry mass to moles (pls check my work)
Rachel Maddiee replied to Rachel Maddiee's topic in Homework Help
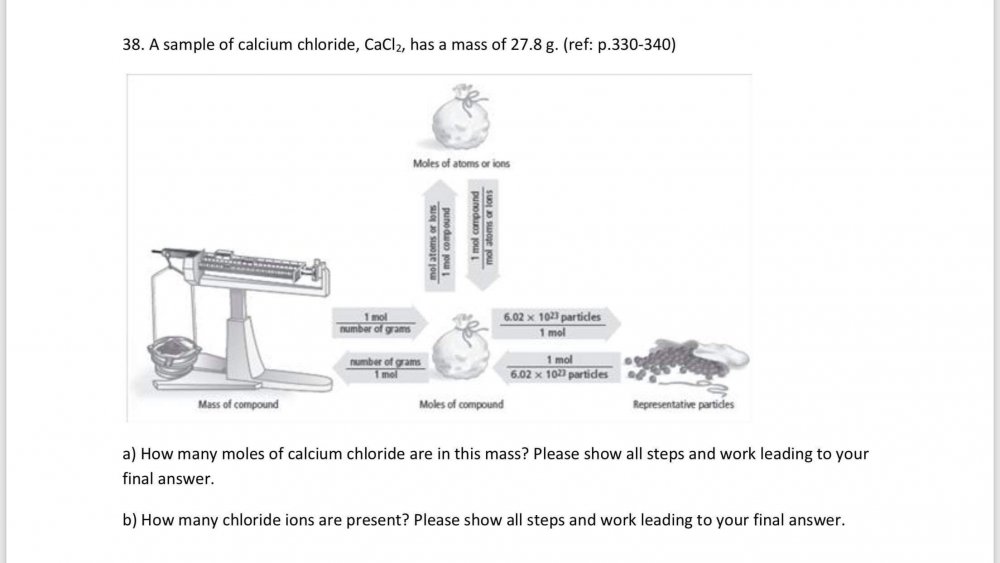
What am I doing incorrectly? Am I missing a step? OK I fixed it mass = 27.8 g CaCI2 Number of CaCI2 in the compound = (unknown) Number of CI- ions in the compound = (unknown) Number of moles = mass/molar mass To find the number of moles of CaCI2 first you find the molar mass of the compound. 1 mole Ca x 40.08 g Ca/1 mole Ca = 40.08 g 1 mole CI x 35.45 g CI/1 mole CI = 35.45 g 1 mole CI x 35.45 g CI/1 mole CI = 35.45 g molar mass = (40.08 g + 35.45 g + 35.45 g) = 110.98 g/mol CaCI2 27.8 g CaCI2 x 1 mol CaCI2/110.98 g CaCI2 = 0.250 mol CaCI2 0.250 mol CaCI2 x 6.02 x 10^23 formula units = 1.505 x 10^23 formula units CaCI2 1.505 x 10^23 x 2 CI- ions = 3.01 x 10^23 CI- ions -

Chemistry mass to moles (pls check my work)
Rachel Maddiee replied to Rachel Maddiee's topic in Homework Help
mass = 27.8 g CaCI2 Number of CaCI2 in the compound = (unknown) Number of CI- ions in the compound = (unknown) Number of moles = mass/molar mass To find the number of moles of CaCI2 first you find the molar mass of the compound. 1 mole Ca x 40.08 g Ca/1 mole Ca = 40.08 g 1 mole CI x 35.45 g CI/1 mole CI = 35.45 g 1 mole CI x 35.45 g CI/1 mole CI = 35.45 g molar mass = (40.08 g + 35.45 g + 35.45 g) = 110.98 g/mol CaCI2 27.8 g CaCI2 x 1 mol CaCI2/110.98 g CaCI2 = 0.25 mol CaCI2 Now from equation No of Chloride ions are : 6.02 x 10^23 x 2 CI- ion = 1.204 x 10^24 CI- ions How is that? -

Chemistry mass to moles (pls check my work)
Rachel Maddiee replied to Rachel Maddiee's topic in Homework Help
I can’t find the typo? And can you please tell me more specifically what is incorrect? thank you! -
-
Is this done correctly? mass = 27.8 g CaCI2 Number of CaCI2 in the compound = (unknown) Number of CI- ions in the compound = (unknown) Number of moles = mass/molar mass To find the number of moles of CaCI2 first you find the molar mass of the compound. 1 mole Ca x 40.08 g Ca/1 mole Ca = 40.08 g 1 mole CI x 30.45 g CI/1 mole CI = 30.45 g 1 mole CI x 30.45 g CI/1 mole CI = 30.45 g molar mass = (40.08 g + 35.45 g + 35.45 g) = 110.98 g/mol CaCI2 27.8 g CaCI2 x 1 mol CaCI2/110.98 g CaCI2 = 0.250 mol CaCI2 Now from equation No of Chloride ions are : 6.02 x 10^23 x 2 CI- ion = 1.204 x 10^23 CI- ions