Rachel Maddiee
Senior Members-
Posts
108 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by Rachel Maddiee
-

Chemistry dimensional analysis (help pls)
Rachel Maddiee replied to Rachel Maddiee's topic in Homework Help
Convert the 100 yards to cm (there are 36 inches in a yard). Divide this by the length of the paperclip 1 yd = 36 in 1 in = 2.54 cm 100 yards = 9144 cm 100 yard x 36 inch / yard * 2.54 cm / inch * 1 paperclip / 3.2 cm = 2857 paperclips -
-
Can someone please help me with this? Im not sure what I’m doing wrong with the units. Convert the 100 yards to cm (there are 36 inches in a yard). Divide this by the length of the paperclip 1 yd = 36 in 1 in = 2.54 cm 100 yards = 9144 cm 100 yds * 36 in/1 yd * 2.54cm/1 in = 9144 cm 9144 / 3.2 = 2857.5 9144 cm/3.2 cm = 2,857.5 cm
-
The de Broglie equation relates to the wave property of matter. λ = h/mv, where λ is wavelength, h is Planck's constant, m is the mass of a particle, moving at a velocity v. h = 6.626 x 10^-34 J•s. m = 96.0 mg v = 30.0 m/s Convert milligrams to kilograms m = 96.0 mg x 1g/1000 mg x 1kg/1000g = 9.60 x 10^-5 kg Calculate the wavelength λ = 6.626 x 10^-34/96.0 x 10^-5 x 30.0 = 2.30 x 10^-31 m Is this right?
-
I’m trying to answer this question using the example in my book but I don’t understand how to go about it.
-
I’m not sure if this is the correct method. a. With the help of the distillation method you can separate and identify the components of the menthol water solution. b. Distillation process is used to separate mixtures from a liquid by exploiting differences in boiling points. The substance with the lowest boiling point will boil first and the fumes will be collected by condensation. c. The advantage of the distillation method is that the heating temperature will decrease at lower pressures and reduce heat energy.
-
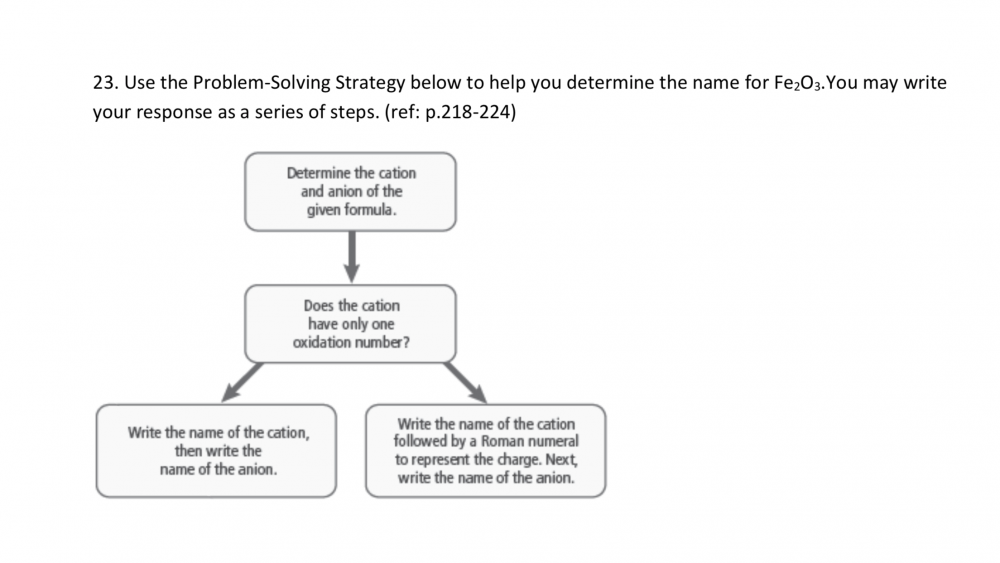
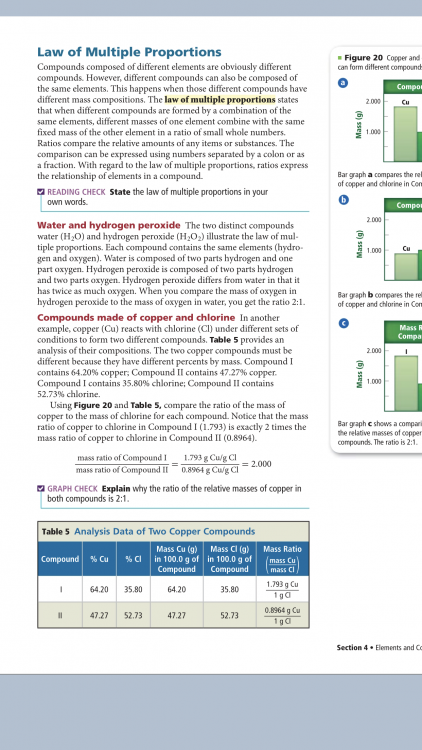
Percent by mass C = 40.60% Percent by mass H = 5.18% Percent by mass O = 54.22% Empirical formula = ? (unknown) Inverse molar mass C = 1 mol/12.01 g Inverse molar mass H = 1 mol/1.008 g Inverse molar mass O = 1 mol/16.00 g Convert the masses to moles. C = 40.60 g/12.01 g/mol = 3.380 mol H = 5.18 g/1.008 g/mol = 5.138 mol O = 54.22 g/16.00 g/mol = 3.388 mol Divide by the lowest, seeking the smallest whole-number ratio C = 3.380/3.380 = 1 = 1 mol C H = 5.138/3.380 = 1.52 = 1.5 mol H O = 3.388/3.380 = 1.00 = 1 mol O The simplest mole ratio is (1 mol C):(1.5 mol H):(1 mol O). 2 x 1 mol C = 2 mol C 2 x 1.5 mol H = 3 mol H 2 x 1 mol O = 2 mol O The simplest whole-number ratio of atoms is (2 atoms C):(3 atoms H):(2 atoms O). The empirical formula of succinic acid is C2H3O2.
-
-
I need help finishing this problem. I’m not sure if the steps are correct of what I have done so far. Percent by mass C = 40.60% Percent by mass H = 5.18% Percent by mass O = 54.22% Empirical formula = ? (unknown) Inverse molar mass C = 1 mol/12.01 g Inverse molar mass H = 1 mol/1.008 g Inverse molar mass O = 1 mol/16.00 g Convert the masses to moles. C = 40.60 g/12.01 g/mol = 3.380 mol H = 5.18 g/1.008 g/mol = 5.138 mol O = 54.22 g/16.00 g/mol = 3.388 mol Divide by the lowest, seeking the smallest whole-number ratio
-

Chemistry: mass to moles to particles conversion
Rachel Maddiee replied to Rachel Maddiee's topic in Homework Help
Is this correct? mass = 37.2 g AICI3 (given) number of ions = AI3+ Ions (unknown) number of ions = CI- Ions (unknown) mass = g/formula unit AICI3 (unknown) The ratio of AI3+ Ions to CI- Ions is 1:3 Molar Mass AICI3 (1 x 26.98 g/mol AI) + (3 x 35.45 g/mol CI) = 133.33 g/mol AICI3 molar mass = 133.33 g/mol AICI3 Divide the given mass of aluminum chloride by its molar mass. 37.2 AICI3/133.33 mol = 0.279 mol AICI3 -
I need help with this question. This is what I have so far mass = 37.2 g AICI3 (given) number of ions = ? AI3+ Ions (unknown) number of ions = ? CI- Ions (unknown) mass = ? g/formula unit AICI3 (unknown) a.
-
a. With the help of the distillation method you can separate and identify the components of the solution. b. Distillation process is used to separate mixtures from a liquid by exploiting differences in boiling points. The substance with the lowest boiling point will boil first and the fumes will be collected by condensation. c. The advantage of the distillation method is that the heating temperature will decrease at lower pressures and reduce heat energy condensed.
-
-
So how should I write it?
-
95 protons
-
#4, 6, & 12
-
What is incorrect?
-
#22? It’s says 4 rules.
-
-
-
-
Did I label everything correctly including steps?
-
Will those numbers be accurate bc some of them were different in the textbook?
-
Density is in mL? g/mL The density of the copper sample is unknown. The copper sample sinks to the bottom and does not absorb any water or dissolve. so all the rise in water level is due to the volume of copper sample. So volume of copper sample = water level after copper added - water level before copper added = ? units? 40 mL - 30 mL = 10 mL Mass of copper = 5g (given) So density of copper sample = mass of copper sample / volume of copper sample = ? units ?? 5g/10mL = 0.5 g/mL Does this work?? In my textbook the units are labeled.
-
40 - 30 = 10 units 5g/10 = 5 units