Everything posted by mundane
-
lewis acid
So, i just learned that because of backbonding in BF3, BF3 is a weak acid. This is due to the fact that B orbital if filled internally with F electrons. Now, if we bring NH3 to make a coordinate bond with BF3, it does. Why? Wasn't the vacant orbital of B already filled during backbonding? I am confused.
-
Forces
So the other day I had a circular tub filled with water. I added a few grams of detergent to it and started stirring with my hands. As expected, detergent tended to settle right at the center of the tub. Was this because of centripetal force? In washing machines, the clothes are spinning but are pushed away from the centre due to centrifugal force. I am not sure. Can someone help me understand the forces acting in both the scenarios?
-
phases and shape of orbitals
I just learned that there are three kinds of overlapping (positive, negative and zero). I don't thoroughly understand the concept of different signs in orbitals and their overlappings. What does difference would it make if the signs are different (negative overlapping happens) when they don't really play a significant role? Could someone explain how waves and orbital shapes along with overlapping in brief?
-
atomic shape
-
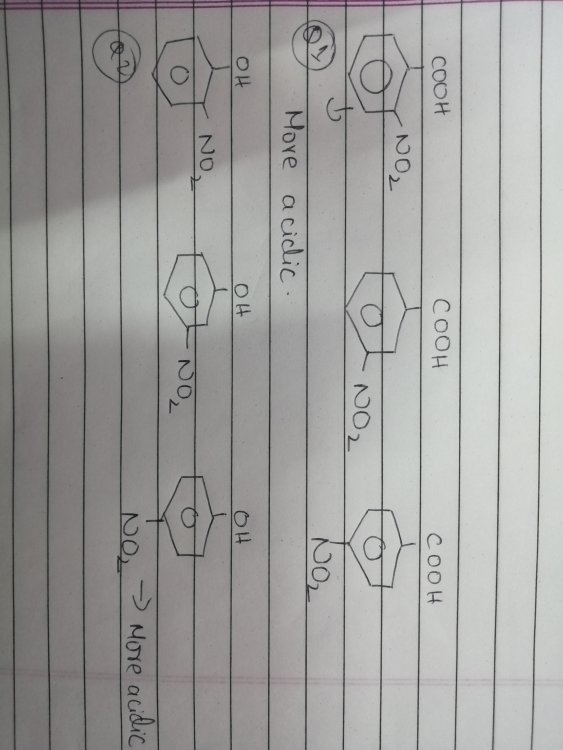
inductive effect and hydrogen bonding
As you see in the first question -NO2 on ortho causes inductive effect and creates a hydrogen bonding with H in COOH and is considered to be stronger acid than others. In the second question, the -NO2 again shows I- and H bonding but is less acidic. I don't get how the latter one isnt the strongest.
-
acidity
I was wondering why we need substances which contain acid in acidity. Can someone help me out?