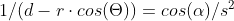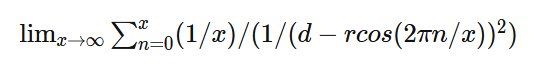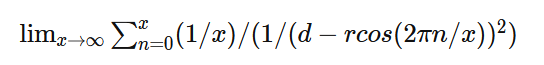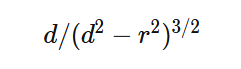-
Simple Unification Theory
I have not found a breakdown of classical shell theory that makes any sense. The original article by Newton is now a screen saver. It refers to corpuscles, circular superficies and sphaerical superficies and a bunch of other stuff that may take a while to figure out in contemporary English. Has anyone got a decent breakdown of classical shell theory that does not involve corpuscles and actually has all the necessary steps? I've looked at a few presentations and they all seem to make giant leaps in thinking without any real justification.
-
Simple Unification Theory
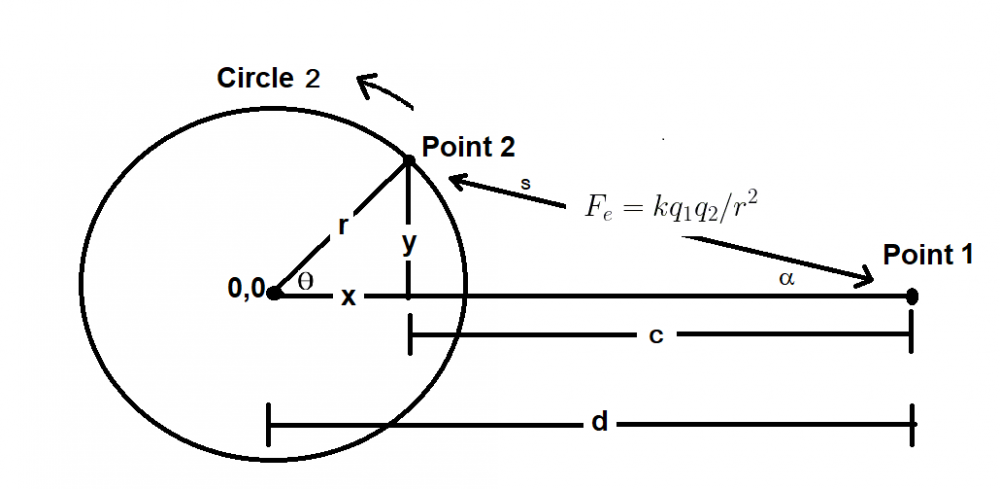
A bit busy with work so this might take a while but I started as you suggested. I only got as far as confirming my approach to calculating the distance in the initial force vector produces the same value as the shell theorem. Namely that with my version on the left and the classic interpretation on the right of that equaity. On to the next step - the bands. Although I don't really understand the necessity of a band in the classic version when the intent is an infinitely narrow band which is a slice through the sphere which is a point on a cross section of a sphere which is my approach. But, after I do my taxes I will resume the process of determining at which point the two approaches differ. And in the meantime if anyone can tell me what's wrong with my far simpler approach I'm still all ears. Ideally if anyone knows of a numerical approximation of the classical shell theorem. Thanks.
-
Simple Unification Theory
Is nobody replying because I haven't explained this properly or because this concept means a lot of physics is wrong? I am reminded of Monty Python - I came here for an argument But, I could be arguing my spare time.
-
Simple Unification Theory
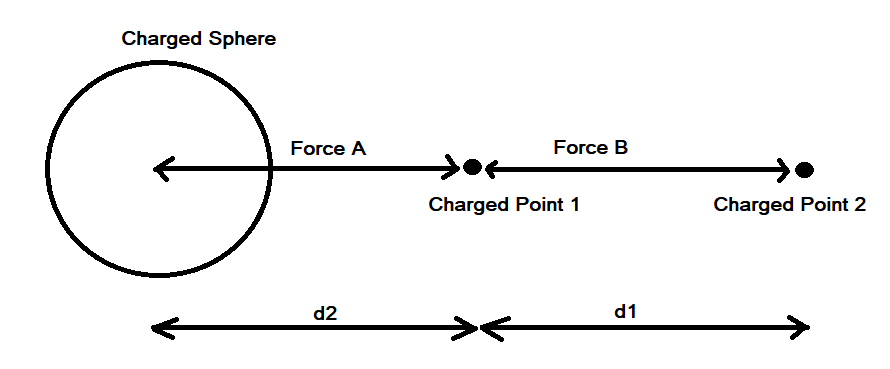
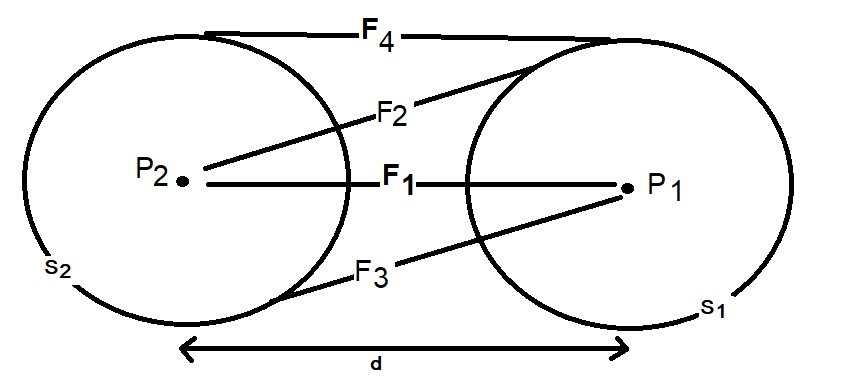
It is confusing. I'm saying that the Coulombic force of q1q2/r^2 does not apply between a point and a hollow sphere. Although conventionally a force calculation between a point and a hollow sphere is approximated as a point and another point and q1q2/r^2 is used. But, if you run a very simple numerical approximation between a point and a hollow sphere where the sphere is represented as n points, using the standard Coulombic equation between the n points and sum the forces it does not equal the force of q1q2/r^2 when the sphere is represented as point. Assuming distances d1 and d2 are identical and the charges on points 1, 2 and the sphere are identical the electrostatic force using Coulomb’s Law between points 1 and 2 does not equal the electrostatic force between point 1 and the charged sphere.
-
Simple Unification Theory
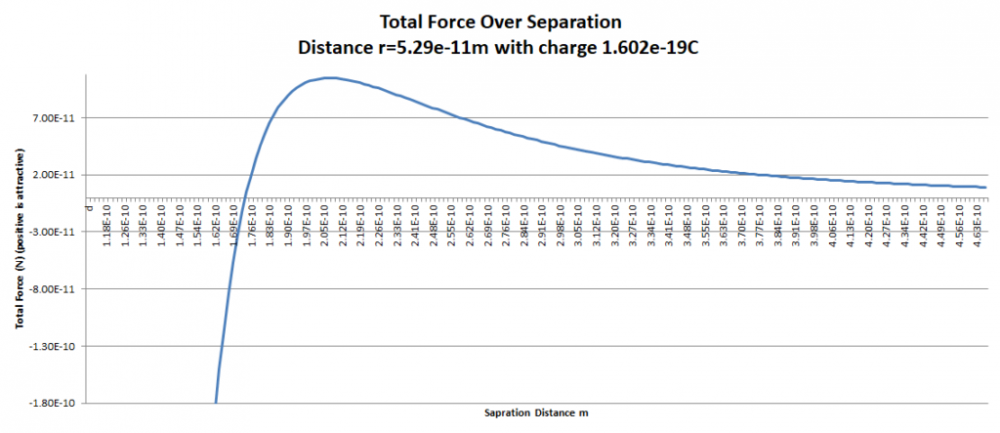
Apologies for the delay – was on the road for a few days. The entire thing is a strange concept. My argument is that a simple numerical approximation using a spreadsheet disagrees with the shell theorem. Below is the approach for the numerical approximation. Also attached is a spreadsheet implementing the diagram. The numerical approximation in the spreadsheet over n rows is shown as a Riemann sum below. Which evaluates to If you use that equation, and not the classic q1q2/r^2 equation, for point to sphere interaction a Hydrogen molecule now has four different electrostatic forces at play, well three in the case of Hydrogen gas. And the weird part - if you graph the net force over distance of all forces you get a Lennard Jones curve. And the peak is about where it should be at stable molecular bond distance and the tail trails off infinitely with an ever decreasing attractive force ( a bit like gravity). Lots of people have looked at this and said it cannot be correct but nobody can tell me what is actually incorrect other than it disagrees with pretty much everything physics is based on. Cheers, Colin. Paper_PtoC_Sample_20210627.xlsx Paper_CtoC_Sample_20210627.xlsx
-
Simple Unification Theory
Hi, Completely new to the forum. Please be kind. Just looking for some feedback on an idea that a single equation to calculate both the force of molecular bonds and the force of gravity is possible if a sphere is not approximated as a point at the molecular level using conventional Coulombic forces. Anyway, my attempt at explaining it is here https://newtontweaked.com/ Thanks in advance, Colin.

ColinJ
Members
-
Joined
-
Last visited