-
Posts
222 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by Yggdrasil
-
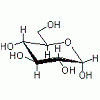
Sizes of human pituitary glands' proteins
Yggdrasil replied to question's topic in Biochemistry and Molecular Biology
Well, the pituitary gland has litterally millions of proteins in it. If you are looking for the size of a specific protein, we could possibly help, but unless you are looking for something more specific, I don't think I could help you. -
Yes, sometimes antibodies cannot distinguish between alternatively spliced versions of proteins.
-
The structure of collagen has been solved and is available through the Protein Databank (PDB). http://www.rcsb.org/pdb/molecules/pdb4_1.html
-

signal transduction for 9th graders
Yggdrasil replied to beachbum's topic in Biochemistry and Molecular Biology
Signal transduction may be too difficult of a subject for 9th graders to work on, though it would depend what your project actually is. Signal transduction involves some fairly sophisticated molecular biology which may be beyond the scope of a high school student. Perhaps if you post more details on your project, we would be able to let you know how feasible it is. -
It makes sense to me that nitric oxide would not be good for the eyes. The relaxation of the muscles around the blood vessels increases the volume of the blood vessels (which is the reason for increased blood flow). The increased volume of the vessels will push agains surrounding tissues, increasing intraocular pressure. Since high intraocular pressure is thought to lead to glaucoma and other eye problems, nitric oxide seems like it would be harmful to eye health.
-
A gene does not necessarily make the same protein in different cells or even within the same cell in different conditions. There is a phenomenon known as alternative splicing can change the sequence of the mRNA transcripts of a gene, resulting in different gene products. Alternative splicing can greatly change the structure and function of various proteins. Many researchers believe that most of the complexity in human beings arises from the alternative splicing of our genes. Probably the best assay to use would be the one Mokele described. If you have reason to believe your gene may be alternatively spliced, you can try to identify variants of your protein by performing 5'-RACE (Rapid Amplification of cDNA Ends).
-
The main ingredient of viagra is nitric oxide (NO). The scientists received the nobel prize for their study of the biological roles of nitric oxide because the fact that nitric oxide acts as a signaling molecule is a very surprising and revolutionary idea. Nitric oxide is a very unstable free radical. Normally, the body wishes to get rid of free radicals as they can cause damage to DNA and other important biological molecules. Plus, signaling molecules, because they need to be transfered between cells, are generally thought of to be stable. So, the fact that an unstable free radical acts as a signaling molecule was quite a revolutionary find. Here's a page with some more information on nitric oxide: http://en.wikipedia.org/wiki/Nitric_oxide I'm sure you could find more information by searching for nitric oxide on google or PubMed.
-
The answer is false because linear dependence doesn't really depend on the number of vectors in a set. You can prove it's false just by showing a trivial counterexample. For instance, in R3 (a 3-D vector space), you can show that the subset {(1,1,0),(2,2,0)} contains less than three vectors and is linearly dependent. On a related note, the converse of this statement is true: given a finite-dimensional vector space, V, with dim V = p, any subset containing more than p vectors must be linearly dependent. To answer your related note, the number of vectors in a basis for your subspace will always be equal to the dimension of the subspace.
-
I've heard the upper class refereed to as yuppies. Also, for the definition of punk, I like Greg Graffin's definition: http://www.badreligion.com/news/essays.php?id=5
-
It mostly depends on the disease, but most of the time you will want the defective gene replaced in all cells of a specific tissue type. Sometimes a small scale approach could work, but sometimes you need a systemic one. For example, type I diabetes is caused by defective islet cells which cannot produce insulin. This is an example of a disease which could be cured by a small number of cells, since it affects only pancreatic islet cells. If you fix/replace enough of them to get the pancreas to produce a normal/sufficient amount of insulin, you've cured the disease. On the other hand, cystic fibrosis, which results from defective sodium channels in epithilial cells (most problematic in the lungs, pancreas, and intestinal tract), requires a systemic approach. Here, replacing/fixing all of the cells would have to be the solution, since the dissease affects multiple parts of the body. Furthermore, if you just fix a few cells, you still get mucous build up because of the deffective cells you didn't fix. Even if you manage to fix/replace all of the lung cells, you still have problems with defective cells in the pancreas and intestines. It would be much more effective to fix the defective gene in the small number of cells in the embryo than it would be to fix a much larger number of cells in an adult.
-

synthesis of meta chloro propylbenzene
Yggdrasil replied to budullewraagh's topic in Organic Chemistry
-

synthesis of meta chloro propylbenzene
Yggdrasil replied to budullewraagh's topic in Organic Chemistry
Chlorine is an ortho/para dirrecting group, so if you chlorinate first, then use f-c alkylation, you would get primarily o-chloro-propylbenzene and p-chloro-propylbenzene. Although you would get some m-chloro-propylbenzene, you'd probably lose a significant amount separating it from the ortho and para forms. So, while the correct answer takes more steps and may have a lower overall yield (I would question whether it does, though), it allows you to easily purify your product. -
Euler's identity is used a lot in solving differential equations. It is also useful because it allows one to express the trigonometric functions in terms of exponential functions.
-
There was actually an article on this in Scientific American recently (in the Apr 2005 issue, check it out on pg 26-27). The researchers actually don't look at the atom, but rather image the wavefunctions of the electron orbitals around the atom. They do this by using a laser pulse to get an electron in a state which is semi-in-place and semi-excited. Apparently, this will cause the electron to emit a plane wave which interferes with the original wave function of the electron. The interference patern causes an emission of UV radiation which the researchers can examine to determine the wave function of the electron. Apparently, this technique is so powerful that it may be able to image molecules during chemical reactions. Very exciting from a chemist's point of view.
-
Look up the definition of e, for example at Wikipedia: http://en.wikipedia.org/wiki/E_%28mathematical_constant%29
-
Economic Left/Right: 0.00 Social Libertarian/Authoritarian: -3.95 Very interesting. Apparently, I am pretty moderate when it comes to economic issues.
-
DNA is not antigenic because antibodies recognize antigens via specific structural features, and DNA fragments from different organisms are very structurally similar. For example, the sugar-phosphate backbone, on the outside of the DNA molecule, is identical in all DNA molecules. Only the base pairs differ between DNA molecules. Since the base pairs are sequestered within the interior of the DNA double helix, specialized structures are needed to access them. Proteins and oligosaccharides, on the other hand, have many structural features which an antibody can use to distinguish between native and foreign proteins/oligosaccharides.
-
Probably the first thing I'd look at is melting point. If the melting point difference between the two compounds is greater than 20°C, it should be easy enough to identify the unknown compound (it is more difficult to distinguish between the two if the mp difference is less; I wouldn't try this method if the difference were less than 10°C). However, after a quick internet search, I could only find the melting point of acetominophen (tylenol): 169°C. So, if you have the equipment to somewhat accurately determine a melting point and the melting point difference is at least 10°C, I would try this.
-
The addition of Br2 to a pi bond does not generate a carbocation. This is shown by the fact that no rearangements associated with carbocations are seen during such reactions. Rather, a bromonium ion is formed. I agree that the OH itself would not act as a leaving group in the final part of the reaction. However, if the OH group were protonated by the HCl, I could see how a bromonium ion would be formed. Cl- is not a good enough nucleophile to displace the oxonium ion in an Sn2 reaction. Rather, the oxonium will leave as water, forming a carbocation at carbon 3. Either the Br or Cl- could form a bond with the carbocation; however, since intramolecular reactions are much faster than intermolecular reactions, the bromonium ion will form before the chloride has a chance to react with the carbocation. Remember, the dissociation of water and formation of the carbocation is the rate limiting step. Therefore, the favorability of the steps following the formation of the carbocation will be determined by kinetics (which reaction goes faster), not thermodynamics (which reaction produces a more stable product). Once the bromonium ion is formed, the chloride can attack carbon 3 and displace the bromine, forming 2-bromo-3-chloro-3-methyl-pentane in which the bromine and chlorine are anti to each other [the product itself will be a racemic mixture of the (R,S) and (S,R) enantiomers].
-
For what oils do you need viscosities?
-
People and person are not pronouns.
-

Explain to me some schools their major aspect.
Yggdrasil replied to ps2huang's topic in Science Education
UCLA is a great school to go to as well, especially if you are interested in biochemistry. I am currently in my junior year at UCLA (major: biochemistry, minor: mathematics) and I would recommend UCLA to anyone. UCLA has made many major discoveries in biology and biochemistry including helping solve the structure of the ribosome and determining the mechanism of ATP synthase. Both UCLA and Berkeley have strong programs all across the board if you are interested in expanding your knowledge beyond just the biological sciences. UC Davis is another good school for bio, although it's programs are more limitted in other areas. Originally an agricultural school, UC Davis has great programs which relate to agriculture -- for example, its plant biology program and its veterinary school. Plus, if you're interested in internships in biotech companies, Davis is near some important biotech firms (including Genentech, which is based in San Francisco [about a 1.5-2hr drive from Davis] and has a plant in Vacaville [about 20min away from Davis]). However, having grown up in Davis, I can say that it's not a very exciting place to live. UC San Diego also has very good science programs and is located near some biotech companies if you're interested in internships. In terms of prestige, UCLA and Berkeley are at the top of the UCs, while UCD and UCSD have a little less prestige. Of course, you can't go wrong with Stanford or MIT either. -
Milk teas with tapioca balls in them, sold under the name "Boba," are pretty popular in Southern California. Not sure how popular they are in other regions of the states.

