Everything posted by mcstroom
-
When indication is there that a organic chemistry (solve the product) rauation is over
i'am simply tying to predict products I'am sorry for the late response I completly forgot about my post here I've got to go sleep right now, so I won't be able to respond for 8 hours so😅
-
When indication is there that a organic chemistry (solve the product) rauation is over
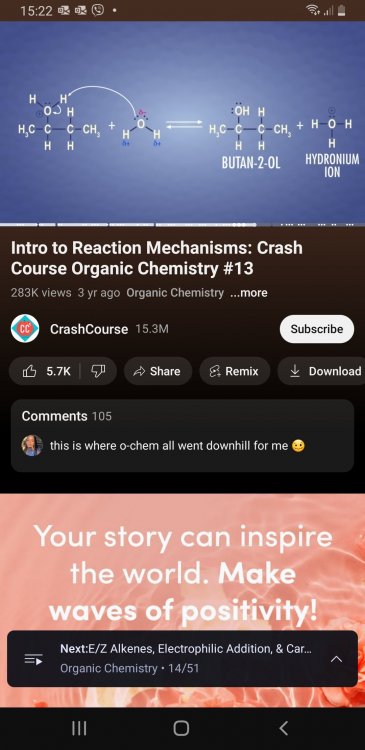
I was not expecting that awnser. It depends on the reaction I am doing. Wow. I'am doing many reactions. There are many. -Alchols recting with acidified di chromate -Alchols reacting with hot sulfuric acid -oxidation and reaction reactions -acetic acid reactions etc The way I understand is that the product that that used to be a reactant and had the nucluphile has to have no formal charges but evreything else that was formed by leaving groups can have a formal charge. How far away I am from being correct
-
When indication is there that a organic chemistry (solve the product) rauation is over
What indication is there that a organic chemistry reactions is finnished
-
When indication is there that a organic chemistry (solve the product) rauation is over
I thought that you keep on doing attacks untill you give evrey atom its proper valance electrons but according to the reaction in the video I saw I was wrong so can someone please help.
-
I'am solving equations that have the following terms in their equation. What should I do
- Frequently appearing problem when trying to solve equations
I attached the wrong screen shot. It was j sho asked that question on reddit.sorry for the confusion- I'am solving equations that have the following terms in their equation. What should I do
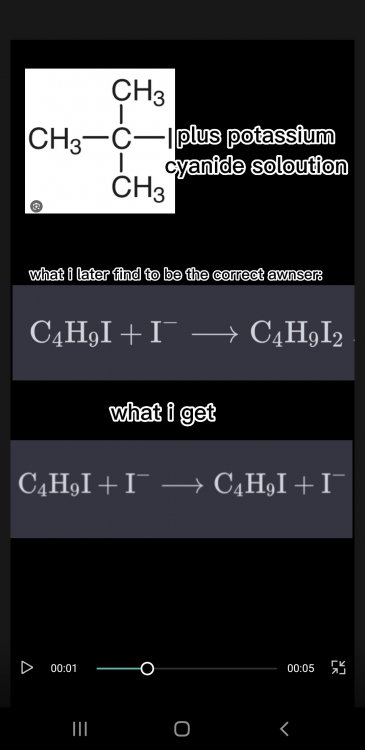
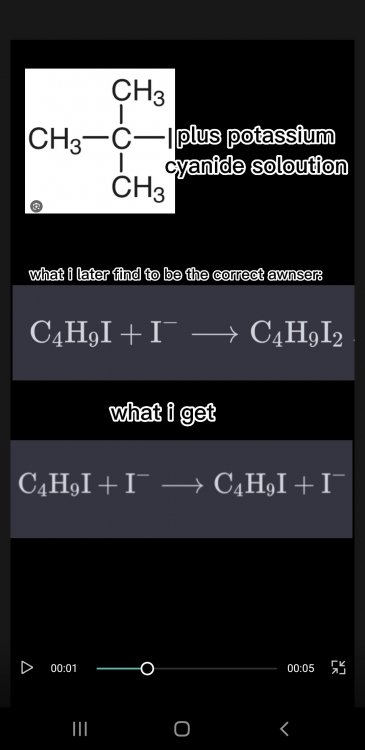
There is only one thing which is stopping me from awnsering them and thats the fact that regarding how my confusion on what to put on the left side and what to put on the right side (of the reactants). I put an example below. Evrey time I solve an eqation I see the the one product is on one side or the first 2 or 3 ekements on the first product are on the second product I'am doing a Btec applied science course and this just the first assigmnt on organic chem its unit 14 A- I'am solving equations that have the following terms in their equation. What should I do
I'am not a bot lol 😂😂 how can I even prove that Iam not one I go to college in the UK I'am not an organic chemistry student so I hope that explains my lack of expertise- Frequently appearing problem when trying to solve equations
How does someone know what to put on the left side and what to put on the right side (of the reactants). I put an example below. Evrey time I solve an eqation I see the the one product is on one side or the first 2 or 3 ekements on the first product are on the second product- I'am solving equations that have the following terms in their equation. What should I do
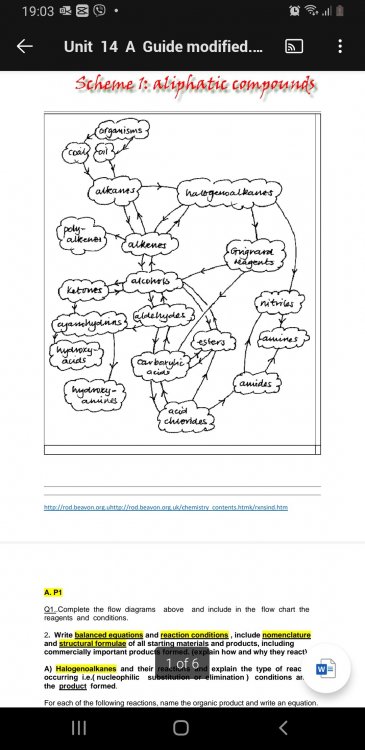
- No one i know in my class knows what this question about the diagram is really asking you to do
- Organkc chemistry help
Thanks for the help , I appreciate it- Organkc chemistry help
No I haven't. I know how to write down the chemical formulae down for alcohols acids etc when they are on there own but when it comes to 2 word esters like methyl ethanoate and proanyl chloride then I don't know.- Organkc chemistry help
I'am struggling to find videos on youtube that explain how to solve chemical equations with organic compounds in them. What skills do I need to know to awnser thses type of questions. T The assighment summed in a few questions: -pentanoic acid + Na2CO3 -methylethyl propanoate + NaOH. -Butanol + propanoyl chloride -Butyl amine + propanoic anhydride. -2-bromobutane + dilute aqueous sodium hydroxide solution/hot concentrated ethanoic potassium hydroxide solution/excess ammonia -butan-1-ol + H2SO4/K2Cr207/ distilling off the product/ H2SO4 /K2Cr2O7 refluxing/ Tollen's reagent with heating. - Frequently appearing problem when trying to solve equations
Important Information
We have placed cookies on your device to help make this website better. You can adjust your cookie settings, otherwise we'll assume you're okay to continue.