Everything posted by Ramil
-
I need help identifying a byproduct of a Hantzsch type reaction
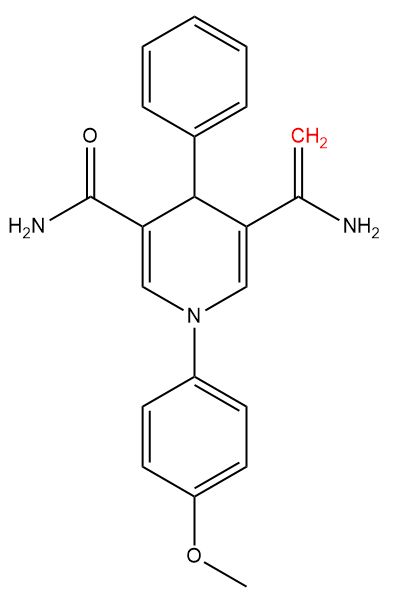
I just want to make sure that the structure you have shown indeed matches with the name you have provided. Actual structure of (5-(1-aminovinyl)-1-(4-methoxyphenyl)-4-phenyl-1,4-dihydropyridine-3-carboxamide) is as following: The two hydrogens attached to the carbon-carbon double bond (highlighted in red) are expected to show a signal around 5~6 ppm.
-
Mechanic of isopulegon
PCC reagent was first developed by Corey group in 1975. You can refer to this paper to see the details regarding its introduction. In regards to my background, I had to solve many Chemistry Olympiad problems back in high-school and I have seen this reagent overwhelmingly frequent. It seems it is used widely. Hope it helps.
-
I need help figuring out the explanation for this question
I agree that there is contradiction and reaction is indeed exergonic (though in addition to being stereoselective). But I think the given question might be problematic and just for the sake of question statement we can not ignore the fact and it is my duty to show the correct application of theory to the given example, which is in this case determination of stereoselective E2 reactions.
-
I need help figuring out the explanation for this question
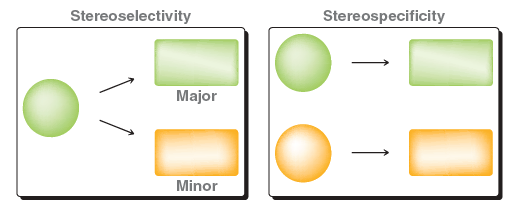
Just because there is one answer allowed does not justify that this reaction is not stereoselective. FYI, E2 reactions can be both stereoselective and stereospecific depending on the number of beta-protons present. In this case, availability of two protons in beta-position results in formation of both cis- and trans-alkenes with trans being the major product. The reactions that yield two stereoisomers with differing amounts are called stereoselective. In contrast, product of stereospecific reaction depends of the configuration of starting material(s). You can refer to following figure from textbook D. Klein to understand the difference:
-
Mechanic of isopulegon
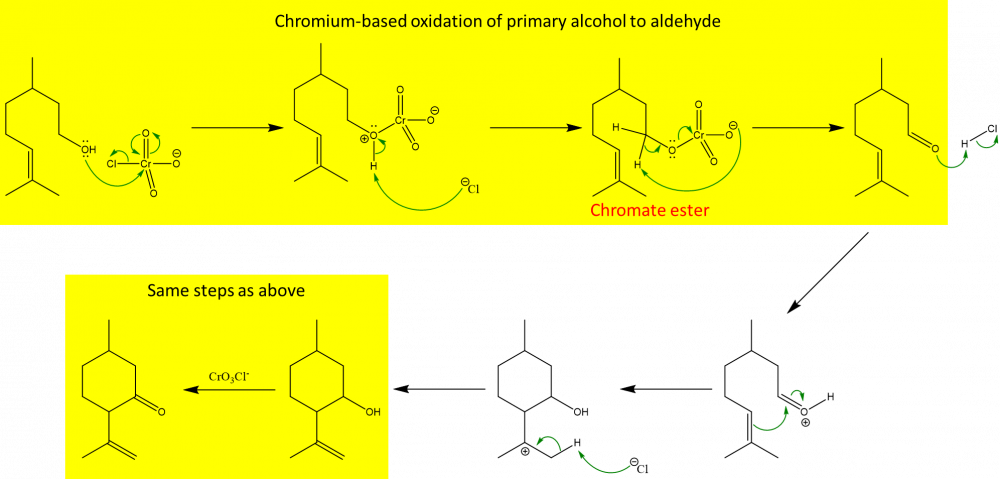
Yes, CrO3Cl- is part of pyridinium chlorochromate (PCC) which is less reactive than other commonly used chromium-based reagents (such as Na2Cr2O7 with H2SO4) and is used in oxidation of primary alcohols to aldehydes (instead of to carboxylic acids)
-
Mechanic of isopulegon
-
I need help figuring out the explanation for this question
This reaction is definitely stereoselective (Answer: C) It uses sterically hindered base, so product is Hoffman product and base abstracts proton from less hindered beta-carbon. But that beta-carbon has two hydrogens and both can be removed to yield cis- and trans-alkenes simultaneously. Among mixture of two diastereomeric alkenes, trans-alkene will predominate. This kind of reactions are called to be stereoselective.