

Sha31
-
Posts
73 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by Sha31
-
-
Mass
in Speculations
Hey there,
By some chance I ended up reading some post of yours from couple of years ago and I noticed your theory is amazingly similar to some of mine conclusions. Though interestingly, your approach and my approach is completely different which makes similarities in conclusions even more intriguing.
I like what you said here:
Vacuum force and anti-vacuum force (matter) waves naturally form planes at right angle to each other (i.e. ‘north to south and equatorial’ or technically' date=' ‘transverse and longitudinal’) the vacuum force is responsible for magnetic action while the matter is responsible for the electric force. The two actions combined cause particles to move towards points of equal density (gravitational action).
[/quote']
...though while you sometimes use concepts of special relativity and vacuum, my conclusions come from classical electromagnetism, mainly applications of Lorentz force, and so instead of vacuum I have Aether. Have a look at it here: http://polarelephant.blogspot.com/
Anyway, about the opening post. I'm not quite sure who said what, so could you summarize quickly what is the argument and what is contra-argument? Additionally, can you explain why would electric and magnetic opposite poles attract, while with gravity like poles attract?
0 -
You measure the charge-mass ratio; also' date=' muons are unstable and will decay into other particles.[/quote']
But how, what exactly can we measure? Can even bubble chamber trajectories tell what's effect of what force? Can even particle accelerators measure the speed of those particles?
And then, I could not google anything about slowing electrons down. I just can't imagine electrons floating by at low speeds, like a meter per second. I'm not sure why, but that just sounds impossible. Hmm?
Also, isn't there some "wave energy" associated with electrons or electron beams, and isn't there some "spin energy"? Then, what if electrons can spin around more than one axis, will the kinetic energy, or angular momentum, of those two spins add? In other words, could there be 3 independent spins, around 3 axes, each contributing to the total angular momentum? And finally, what if muon is just an electron with larger angular momentum than "normal" electrons, could you tell the difference?
0 -
Well just solve the equation and plug the numbers in.
[math]v=c \sqrt{1 - \frac{1}{(E_k/m_oc^2 +1)^2}}[/math]
So for 250 MeV of kinetic energy' date=' an electron would be moving at 299,791,834 m/s or 0.99999792 c
The same equation will work for the other speeds, just plug them into the equation I linked to.
250 MeV [93,835,378,660 m/s]
v=299,792,458 * sqrt( 1 - 1/ (250,000,000/510,998 +1)^2 )
= 299,792,458 * sqrt( 1 - 1/ 240,333.9883 )
= 299,791,834 m/s
30 keV [3,248,528 m/s]
v=299,792,458 * sqrt( 1 - 1/ (30,000/510,998 +1)^2 )
= 299,792,458 * sqrt( 1 - 1/ 1.120864 )
= 98,444,785 m/s
1 eV [593,097 m/s]
v=299,792,458 * sqrt( 1 - 1/ (1/510,998 +1)^2 )
= 299,792,458 * sqrt( 1 - 1/ 1.000003914 )
= 593,096 m/s
I got different result for 30 keV, but the same result for 1 eV, as before. Anyway, when you measure some particle has 250 MeV energy how do you know whether it is fast electron or slow muon?
0 -
What do you mean out of range? You need to use the relativistic equation for kinetic energy if you want to go at such high energies.

This one? Ok, what is velocity equal to?
0 -
How the charge of an electron was first measured:
http://en.wikipedia.org/wiki/Oil-drop_experiment
You might wish to read a bit more about that.
In an electrons rest frame it is moving at 0 velocity, this all becomes a bit complicated as we need to take into account quantum mechanics and we need to talk about electrons as wave-particles which don't really have a well defined position or velocity.
In your maths to find the velocity, write down the full units at each step (mass in eV is actually eV/c^2), I don't have the time right now to work out whether you've done it right but if you put in all the units it's far easier for someone to just scan it to tell if it's correct. Also remember that if the velocity is relativistic you need to use the relativistic equations, not Gallilain.
E = 1/2 * m * v^2
30,000[eV] = 0.5 * 510,998/299,792,458^2[eV/m/s] * v^2[m/s]
v = sqrt(30,000/2.84281e-12)[m/s]
= 3,248,528 m/s
1 eV, v= 593,097 m/s
30 keV, v= 3,248,528 m/s
250 MeV, v= 93,835,378,660 m/s
Ok, this looks better, but 250 MeV is out of range, so what are the minimum/maximum electron energies we can produce/measure?
Merged post follows:
Consecutive posts mergedAn individual electron can have an arbitrarily small speed.
This sounds very strange to me. For some reason, I simply can not imagine electrons move at any slow speed, like a meter per second or slower? What is the process and technology to slow down electrons? What words to google?
0 -
They don't have the same velocity. Nobody said they had the same velocity. They have the same energy. an electron' date=' or a proton, or any other particle with a fundamental charge, will gain 1 eV of energy when the potential difference is 1 Volt.
[/quote']
Ok. Next question. Can you explain how can electrons be slowed down and just how slow can they go? I mean without jumping from atom to atom, but moving in a beam through air or vacuum. Also, what happens with special relativity and all that mass increase stuff as electrons close to the speed of light, do they kind of start to look like muons due to their newly gained mass?
No, energy is not mass*velocity, that's momentum. E=mc^2 The electron mass is 0.511 MeV, if you express it in terms of energy (i.e. you multiply by c^2). That's a common shortcut/notation in physics.
These are all commonly used, defined terms. You don't get to redefine them on a whim.
I was talking about kinetic energy since that is how eV is defined. Anyway, what is the velocity of an electron with energy of 1eV, 30keV and 250MeV?
E = m/2 * v^2
30keV = m/2 * v^2
30,000 = 510,998/2 * v^2
v= sqrt(30,000 / 255,499) = 0.342662 ??
That's wrong obviously, and what happens to units?
Can someone do it properly?
0 -
This is very interesting, if it is true and that a batch of super-heavy elements is on the brink of being found this could dramatically change chemistry. What will be even more awesome is if and when we get to explore the properties of this newly discovered super-heavy element. Maybe it will turn out to be the philosopher's stone!!!

White powder gold - monoatomic gold, they call "philosopher's stone", for some reason. I have no idea what is claimed this thing can do, but the existence of this substance and the story of its discovery seem to be quite true.
0 -
Well' date=' you are doing it backwards. You calculate the kinetic energy from the work done on the electron (as a charged particle, the mass never figures into the equation). Then you calculate the velocity it will be at to have that kinetic energy, since you know its mass. Why? Because it is easier that way.
[/quote']
Maybe I wasn't clear. Yes, I agree definition works for any charge, but I don't think it correctly describes gravity force and that is a proper definition for mass. -- Electron volt is defined as a difference in 'kinetic energy', and kinetic energy is a product of two physical properties, mass and velocity. It just so happens some imaginary relation named "work" is defined as exactly this energy difference, but that in no way avoids the fact this energy is actually "made of" mass, so 'mass' ends up being defined by using concept of "mass". There is no definition, or unit, nothing like this in the whole physics, as far as I know, it's just wrong, like chicken and egg.
An electron volt is not used to measure the mass of an electron. It is a unit of energy with which the mass of an electron can be expressed in, just like meters, feet, and cubits are used to measure distance.
Ok, after reading that article linked below, I think I can answer that question now - eV actually gets divided by c^2. So, it's just a shortcut to just write "eV" instead of "eV/c^2". Funny. Well, this surely answers one of my questions, but I'm afraid it opens a few new ones, like - just how slow electrons can go and what happens with all that mass increase stuff as they close to the speed of light, do they kind of start to look like muons with their newly gained mass?
http://en.wikipedia.org/wiki/Mass
- "The electronvolt (eV) is primarily a unit of energy, but because of the mass-energy equivalence it can also function as a unit of mass. In this context it is denoted eV/c^2, or simply as eV."
An electron has a mass of 9.10938215×10−31 kg or 8.18710414 × 10-14 joules or 510,998.903 electron volts. As you can see, an electron volt is the only of these units that is not obscenely too large to measure the mass of an electron. Actually mega electron volt is the one that is exactly right for particle masses.
Ok. Can you tell what is the velocity of an electron with energy of 1eV, 30keV and 250MeV?
0 -
Why are you interested in the velocity?
In any case' date=' you can get the velocity by (for non-relativistic velocities) taking [math']v=\sqrt{2 \cdot KE/m}[/math], where KE is the kinetic energy, and is equal to the work gained by an electric charge falling through 1 volt. Calculate this for 1 eV and the mass of an electron, and you get the electron's velocity at 1 eV. Calculate the same for a proton, and you get the proton's velocity at 1 eV. The proton will be moving slower.
That is exactly my point. I'm interested in velocity because that is one of only two variables defining kinetic energy, second being mass. Yes, the proton will be moving slower and for equation to work, for energy to come up the same, we have this larger mass so the product can give the same result: mass*velocity. And there it is 'mass' right there in the definition of electron volt, as one of the two properties defining 'kinetic energy'. The other thing is how can eV = kg? How can unit for energy substitute unite of mass, what happens to velocity?
It doesn't matter. If you know the force and distance, you calculate work that way. If you know the mass and change in velocity, you calculate it the second way.
Yes, but work is imaginary concept, some useful relation, while distance, force and velocity are true properties of the real world. Mass is also supposed to be one of these true and measurable properties, like charge, which is what I kind of question here.
Because that is not specific. You are not specifying a gravitational force, so the energy could be anything.
But, charge in coulombs is not specific too, and is experimentally determined, so naturally, the mass was involved in the measurements of these forces via F=ma. Don't you see something is strange about defining gravity force (mass) with electric force? It's like defining 'charge' with free fall.
0 -
You're moving the goalposts. I said you don't need the mass to calculate the energy. I did not say you could also calculate the other terms you have listed.
The point is not to skip any derivation steps' date=' so to see if the definition is self referencing, if it is circular. When you say "work", that automatically involves force and distance, which automatically involves acceleration and velocity. Velocity is the variable we are after.
And while there are shortcuts when stuff gets canceled and some things end up to be equal, for the point I'm making we have to realize what came first, what means what, what is a shortcut, assumption or derivation and what is actual definition.
Circular definition and self-reference:
"chicken": thing that comes out an 'egg'
"egg": thing from which 'chicken' comes out
So, what came first, work or velocity?
[img']http://upload.wikimedia.org/math/9/a/e/9aeac7ca01e03ffd4b80c513dbeb1b6a.png[/img]

No mention of mass. Why is it not necessary? Because for a given force, a larger mass means the acceleration is smaller and it takes a longer time, and the effects cancel.
Ok, distance is constant, force is constant, so work done is the same. But, how can objects have the same velocity after traveling the same distance if acceleration was different?
In any case, my point is that it makes no sense to say electron mass is 0.511MeV if "mass" itself is involved in the definition of electron volt. The other thing is that mass is NOT energy, mass*velocity is, so how can unit of energy replace unit of mass when it involves one more variable? It's as if some velocity is assumed or suggested in the same time with this "mass".
That's because the fundamental charge is an experimentally determined quantity. The definition, i.e. the equation, is still exact.
Why not define mass as amount of work done by gravity force on an object free falling through the distance of 1 meter?
0 -
electron-Volt works for any charge. KE = qV
That definition is specifically using electron to define this particular amount of energy' date=' hence the name. Do you think you can take a proton and it will gain the same amount of energy as electron?
It measures the amount of work a field does on the charge.
So, we have some distance and we have some force, it also means we have some delta-time, right? What exactly is the equation you suggest here to calculate energy, work, acceleration, velocity and distance from this, without using mass?
If a fundamental charge has moved through a 1 Volt potential difference, it will have gained 1 eV of energy.
Yes, fundamental charge - "electron", not any charge. Gained *kinetic* energy, not any energy, since mass is constant that can mean only one thing - gained velocity. So, how exactly do you calculate this difference in energy independent of mass variable?
This is exact, because it's by definition. F=ma doesn't really enter into it, because of the charge has not accelerated to the correct speed, then it will not have gone through a 1 V potential difference. This is trivially derived from the definitions of potential difference and electric field.
I say it is not exact and that it very much depends on mass and F=ma. There is a circular logic for confirmation in your reasoning again, so it wold explain a lot, and it would be the most helpful, if you could just print down those trivially derived equations you're talking about?
http://en.wikipedia.org/wiki/Electron_volt
- "The electron volt is not an SI unit and its value must be obtained experimentally."
0 -
The electron's mass has nothing to do with an electron-volt. You can use any particle with a unit of charge for that.
Did you say:
- "An electron-volt is the amount of energy an electron gains.."?
To find out that amount of kinetic energy (acceleration and velocity difference) you have to know the 'mass' to start with' date=' because of: F=ma. Beside that, this acceleration will not be uniform and the result will contain error, it's approximation, so this is really terrible definition of mass (energy), it's self-referencing (circular) and it's non-exact.
They have relativistic mass (also known as energy) but have no rest mass.
The equation for energy in terms of rest mass and momentum is,
[math]E^2 = m_0^2c^4 + p^2c^2[/math]
Obviously, [math]m_0 \neq m[/math] except at zero velocity. The advantage of using this formula is that it makes explicit which portion of the energy is due to the rest mass and which portion is kinetic energy.
Some terms, like 'relativistic mass' are defined differently here:
http://en.wikipedia.org/wiki/Relativistic_mass
But, that still solves nothing, it's still circular. Define "p"? Define "m0"?
How did we ever measure and conclude what is electron mass? Can someone point to a couple of different experiments that confirm these numbers for electron mass?
Well they could have different potential energy (eg one could be closer to a proton than the other), but if you mean other than that, then no.
Ok. From electron microscopy we know there are high-energy and low-energy electrons. It is the conclusion then, this energy difference is solely due to electron velocity? But, how is it possible then to make electrons move slower than the speed of light and then even slow them down some more? What is the velocity of an electron with energy of 1 eV and with 30 keV?
0 -
Hm, the thing is that all of these are units of energy. An electron-volt is the amount of energy an electron gains falling through 1 volt, and is particularly useful for particle accelerators, a Joule is what we use to measure the energy of normal sized objects, and mass is related to energy via Einstein's equation. Any of them can be used to measure energy, and people go with whichever is most convenient.
Ok, but there is a problem. The definition is circular. Electron volt, the amount of energy an electron gains falling through 1 volt will depend on electron mass, hence this definition is unsuitable to be defining "mass" as it requires 'mass' to already be defined. Similar thing you can see with this equation for 'massless particles':
p= E/c
but...
E= m*c*c
...so, again:
p = m*c -> p = m*v
Without mass energy is zero, without mass momentum is zero.
Thus, if photons have energy and momentum they must have mass.
Unfortunately, from all this it is still kind of unclear if 'mass' is a real property, do gravity fields really exist, or perhaps gravity force is just some side-effect due to motion of charges, effect of superposition of electric and magnetic fields and their kinematics/dynamics, their kinetic energy. But, anyhow, the real question is this: - can electrons traveling a straight line with the same velocity still have different energies due to some vibration, spin or something?
0 -
Well, the "for dummies" approach is to just put it into Google Calculator. Also works as the "for lazybums" approach.
That is not sufficient then. Can you explain how do you equate kg and eV? How come mass is expressed via some property of electric charge? What gravity field has to do with electric field and its kinetic energy, what is the relation?
0 -
A red photon will have around 2 eV of energy (1240 nm will give you 1 eV)' date=' so the experimental limits exclude this by many frazillion standard deviations.
[/quote']
Thank you, I would have no idea how to even start converting that. Let me just note how that mass (energy) is right in the correct range of what would couple of electric fields have, like electron and positron.
Now, we can have different energy photons, and IF we interpret mass in electron volts we could just as same say these oscillating electromagnetic fields (photons) actually increase in mass. But, we do know that in this case it is not mass that is increasing, we know it is the frequency/amplitude. In other words, it is the increase in some 'perpendicular velocity' or oscillation that brings in this extra energy - kinetic energy - momentum.
Anyway, this logic, if correct, I want to apply to single electrons and see if we can indeed distinguish between electron kinetic energy (vibrations, oscillations, spin) and electron mass.
At low energy' date=' energy scales with the square of the speed, i.e. KE = 1/2 mv^2, so speed and energy effects don't scale the same way.
[/quote']
Ok. Can we have two electron beams with two different energies without changing the number of electrons emitted? In other words, can two electrons traveling a straight line, and having the same velocity, can they still have different energies due to some vibration, spin or something?
0 -
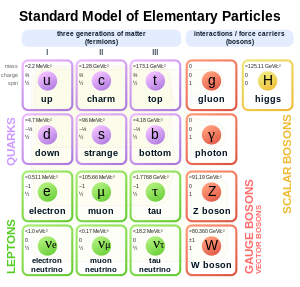
Also an electron is made up of a quark and anti-quark: a Down quark and an Anti Up quark. The Down quark has a charge of -1/3 (e), where e is the charge of an electron (in absolute value) and the Anti Up quark has a charge of -2/3 (e), giving you -1 (e).
If you can not observe these quarks separately, and their charge sums up to one electron, then how do you know that is actually not electron? But, electron is elementary particle, it is not made of anything, or so it would seem:
http://en.wikipedia.org/wiki/Elementary_particle
And P = E/c and E = hc/lambda, where lambda is the wavelength of the photon of light, so plug in the values and you will get the answer.
Also, F = (k * Q*Q')/r^2 r and the electric field is F = Q E, so Q' does not mean its an electron or whatever, its the value of charge of the particle you are talking about. This is usually also for point like charges. So for the case of an electron it has a certain charge (e) you plug that in for the value of Q' and Q is the "test charge" at which you are looking at the electric field.
What I'm trying to say is that electric field is no different thing from electron. Whenever you have some electron you can only know it's there indirectly by probing this electric field. You never actually observe and measure this "ball" we call 'electron' and is supposed to be in the center of this electric field. For all it matters we can take away what has NO SIZE and then we are still left with our field and its charge, we can call it 'electron', give it momentum (mass) and nothing has changed with experiments or equations. Unless, of course, there exist even smaller elementary amount of el. charge.
Merged post follows:
Consecutive posts mergedElectrons and positrons, when put together, will annihilate each other producing distinctive EM waves. How do you intend to keep them from annihilating? How will you explain the strong and weak nuclear forces with them?They do not really annihilate, they form photons (EM waves/radiation). This is confirmed by inverse process, "Pair production", which does the opposite - it splits this electric dipole (photon) into two monopole electric fields, positron and electron.
http://en.wikipedia.org/wiki/Pair_production
http://en.wikipedia.org/wiki/Pair_annihilation
Superposition of positive and negative electric fields, neutralizing each other, would sure explain how can photons be made of electric fields and still have zero net charge, right?
Electron and positron are trying to stick together, but due to their magnetic fields, instead of orbiting, they end up spiraling each other in some linear direction describing double helix, and there it is your transverse EM wave.
http://en.wikipedia.org/wiki/Electromagnetic_radiation

It's interesting Wikipedia even marks the graph with "+q" and "-q".
I'm not aware of any effects of strong and weak nuclear forces with photons and EM waves.
Merged post follows:
Consecutive posts mergedCharge is a property. An electron possesses elementary charge. It is not, itself, elementary charge.Ok. Yes, I agree.
I want to suggest that electric field is elementary entity and that electron, as elementary concept, is just an illustrative representation of this field, it's kind of the same thing. I say, fields can exist on itself (given the medium), like solitons, whirlpools and tornadoes that do not have some particle in the center of the field, but the whole dynamics of it acts as an entity itself.
Muons and pions are trivially observed in bubble chambers.
Cool.
Now, how do you know muon is not electron? According to that table above the only difference is in mass, but would not electrons with high enough frequency (energy) be indistinguishable from muon? In other words, what is the difference between mass given in electron volts and electron energy/frequency or kinetic/wave energy?
How do you differentiate what is mass, what is energy and what is plain velocity?
A zero mass is required by electrodynamics and relativity, and those theories work at very high levels of precision. There have been attempts to measure a mass, and they put very stringent limits on it
http://silver.neep.wisc.edu/~lakes/mu.pdf
http://www.aip.org/pnu/2003/split/625-2.html
The momentum of a red photon is about 2.85e-19 kg-m/s
According to p= m*v, mass of that photon is about: m= 2.85e-19 / c
How much is (2.85e-19/c)kg in electron volts?
0 -
Right. It is the smallest charge that we can separate from a matter and operate with it - accelerate in accelerators, TV tubes, electronic lamps, etc.
Quarks were invented (not directly observed) in order to:
1) explain some scattering experiments with heavy compound nuclei,
2) squeeze the quarks into a certain group of symmetry that require at the same time fractional charge of quarks (3 quarks in proton, neutron, and two in mesons) and impossibility to observe a quark in a free state. The strong interactions are so strong that one cannot separate things in a compound system without creating new coupled quarks. It is such a model in theoretical physics. Just keep in mind that strongly interacting things sometimes cannot be separated, like phonons from a solid. They are some quasi-particles - elementary excitations of compound systems.
We can make proton beams that are beams of coupled together quarks.
Quarks are always bound, mesons are well observed as free.
Ok, thank you, that makes a lot of sense to me. Now, if I have a theory that all composite particles like protons and neutrons are actually made of assembles of positrons and electrons (elementary el. fields whose motion/spin causes magnetic fields), could you refute that?
0 -
Yes. If you srtip many electrons from a body' date=' it gets positively charged due to non-compensated amount of positive charge of all nuclei and the amount of remaining electrons. This happens all the time when wind blows and separates charges between the Earth and clouds. Sudden recombination (neutralization) of separated charges is a lightning.[/quote']
Ok. But electron is elementary particle. It is "elementary charge", i.e. the smallest, indivisible amount of charge that can exist, right? Therefore, positron is all that too only with positive charge, yes?
Are there some quarks, or whatever particles, that have smaller amount of charge than electron? If yes, then why are they not the 'elementary charges'? If not, then how do you know your quark is not actually an electron/positron?
We can make a beam of electrons, can we make a beam of quarks?
Can we observe quarks, or say muons, in bubble chamber?
Merged post follows:
Consecutive posts mergedFor a massless particle' date=' p = E/c
Hit an atom with a photon and it will recoil. That's the basis of laser cooling, which was the subject of the 1997 Nobel prize.[/quote']
Ok. How do we know (measure) photon has no mass?
What is the value of photon momentum, for say red light?
All freely observable particles come in units of the fundamental charge. So an antiproton and e.g. a negatively charged pi meson all have the same charge, and this is confirmed to the level that we can do such experiments.
We know the antiproton does not contains electrons because physicists have been successfully doing physics for a while. For an antiproton contain an electron would violate a large number of laws of physics and contradict a whole bunch of experiments. If that sounds snarky, I apologize, but this encompasses decades of physics research and it's as if you were asking, "How do you know you haven't just gotten it all wrong?" (The answer to which is "Because it all works")
Ok, let me rephrase.
You know proton is not made from positrons, you know it is made of quarks and its positive charge comes from quark's electric field instead, i.e. magnitude of electric charge. So, basically I'm asking what is the difference between quarks and electrons/positrons? And, what is the equation that describes electric and magnetic fields of quarks?
0 -
What if you have an antiproton? Or one of a number of negatively charged mesons?
Ok' date=' but how do you know antiproton does not contain electrons?
What is the magnitude of electric charge of meson and antiproton?
Energy content and momentum ≠ mass
Photons have energy and momentum, but no mass
Momentum implies mass: P= m*v.
Without mass momentum is zero, right?
What is the value of photon momentum?
How do we know (measure) photon momentum?
0 -
In the above context they look interchangeable.
But you should think of the charge as a property of an electron, it is the property that allows it to couple with the electromagnetic field.
The formula you give would also work for spherical charge distributions, i.e. not single electrons.
I said nothing about an Aether.
Ok.
The electric field is not the same as an electron. Electrons act as point-like sources of the electric field.
The electron has properties that are not shared by the electric field. A classical example would be mass. The electric field has no mass. Electrons do.
The only example I can think of where electric fields have no mass is 'photon'. What other example is there of electric field that is actually not an electron? Also, it turns out fields do have mass: - "... and the field has such familiar properties as energy content and momentum, just as particles can have."
0 -
No. Electron and positrons have the property we call electric charge.
The charge is the reason why they interact electromagnetically.
So' date=' "electron" and "single negative charge" are different things?
F= k* q1*q2/r^2
"q" stands for CHARGE, it represents electric FIELD, and we use it to calculate force between two ELECTRONS. The three words seem to be quite interchangeable. In fact, I can not think of any case where there is an electric charge, that does not have electric field, which is not a part of some electron. Numerical value and location for all three of these "things" seem to be in the same spot, sharing the same place with that "q". Why is this?
The electric field should be viewed as (part of) a medium in which electric interactions are transmitted. It is how electrons in a metal "talk to each other".
I know people have thought about electrons as a kind of solition "localised lump" in the electromagnetic field in higher dimensions. But this is not the standard view point.
Aether and solitons, I like that.
At the energy scales of everyday life, all the electric and magnetic fields are due to electrons.
But electrons are not the only charged fundamental particle.
So, the only difference between 'electric field' and 'electron' is that in the case of electron there is some "ball" in the center that has no size?
0 -
Gravity pulls on an object, which speeds up thus gaining kinetic energy.
Like-charged particles repel, which seems to be a method of energy production.
What am I missing?
Field potentials, i.e. potential energy.
It's all fields. Gravity, electric and magnetic fields. They have their potentials that drop off with distance by inverse-square law. So, gravity pulls on an object, which speeds up, thus gaining kinetic energy, but also losing potential energy, hence energy is conserved. However, this does not really explain what in the world is this potential energy and where does it come from. Its a 'property of fields' - I think of it as spherical space-density gradients around charges and masses, kind of like curvatures in space-time.
0 -
Ok. Electrons, whatever they are, also act as sources of electric fields, so would you agree 'negative charge' is the same thing as electron, and that 'positive charge' is the same thing as positron? Then, what is the difference between electric field and electron? And, could there be electric field which is not in the same time a part of some electron/positron (charge)?
0 -
1.) What is the difference between electric field and electric charge?
2.) What is the difference between electric field and electron/positron?
3.) What is the difference between electric charge and electron/positron?
4.) Is there an electric field which is not the field of some electron/positron?
0

Electric field, electric charge, electron and positron.
in Speculations
Posted
I don't understand how that can slow down electrons. "Thermal" is just a description of kinetic state of some medium. Higher temperatures correspond to higher atom velocities or higher molecular vibrations/oscillations. Low temperature decreases velocities, but I don't think electron velocity changes, just this velocity of atoms or molecular vibrations. Electrons need their velocities to stay in their orbit, classically speaking. -- But anyhow, this is the question: how can electron microscopes produce different electron energies, how do they emit slow electrons?
Ok, so there is south and north magnetic pole, and whatever the orientation we can change it in arbitrary direction, yes? In fact, we should be able to spin this electron by influencing this magnetic dipole moment, just like electric motors do, and when we turn our spin induction magnets off, the electron should continue to spin, right?