-
Posts
54722 -
Joined
-
Last visited
-
Days Won
322
Content Type
Profiles
Forums
Events
Everything posted by swansont
-
Since a mole of an ideal gas at STP has a volume of 22.4 L, the lift is about 1g per liter under those conditions.
-

Mathematics is a Fairy Tale.
swansont replied to Willem F Esterhuyse's topic in Analysis and Calculus
! Moderator Note Rule 2.12 We expect arguments to be made in good faith. Honest discussions, backed up by evidence when necessary. Example of tactics that are not in good faith include misrepresentation, arguments based on distraction, attempts to omit or ignore information, advancing an ideology or agenda at the expense of the science being discussed, general appeals to science being flawed or dogmatic, conspiracies, and trolling. Nebulous claims such as this do not make for a good-faith discussion -
And the connection to cold is? This is your proposal. You need to share the details. You can have a high-temperature sample of gas with a large mean free path or a small one. Same for low temperature. Can you clarify this? Radiation can be heat flow, and radiation can cause changes in translational KE. Photons have momentum.
-
Can you explain what you mean by this? This is something new you’ve introduced, with no foundation.
-
Can you add coldness to something that has its maximum amount of coldness? Why not?
-
I recall someone at a science communication conference describing geeks as people who value knowledge more than politeness. That we (I am a geek) don’t mind being corrected because it means we have added to our knowledge. There are some with flipped priorities - they consider being corrected to be rude, with no regard to the veracity of the original claim. Correction just isn’t done, or requires a lot of tact. Scientists and those interested in science discussion, tend to be more in the geek camp than not. There’s no malice assumed when incorrect information is upgraded with better information. For the non-geek, there may also be a matter of projection. One might assume malice if one is prone to being malicious in showing up other people. There are, after all, people who are smart and like nothing more than lording that over other people. I think they tend to belittle others in doing so. A difference between “that’s wrong” and “that’s wrong, you know-nothing imbecile” (aka pushing yourself up by putting others down). I was fortunate in my career in working with lots of smart people who understood there were things they didn’t know, so they didn’t fall into this camp. Most were comfortable in their geekdom.
-
Also: Why would people who think money is speech come up with convoluted ways to donate more money to the people they support?
-
PAC donations are separate; they don’t go to the candidate’s campaign. And above I incorrectly said election cycle, but the limits are per election - the limit applies separately to the primary and general elections
-
Yes, you can contribute directly to a campaign. But that’s limited to $2900 per election cycle for federal office (the amount can be adjusted each election cycle; originally it was $2000 in the 2002 legislation that “reformed” the system) https://www.fec.gov/updates/fec-announces-2021-2022-campaign-cycle-contribution-limits/
-
It’s called UTC
-
Yes. It has already been pointed out that one does not generally refer to textbooks of 100 years ago but uses current ones as much as possible, because mainstream science is the science of today, not the science of history. It’s not static. We learn things. Language evolves. A work dated 1904, for example, cannot reflect fully modern notions of energy, since it predates relativity.
-
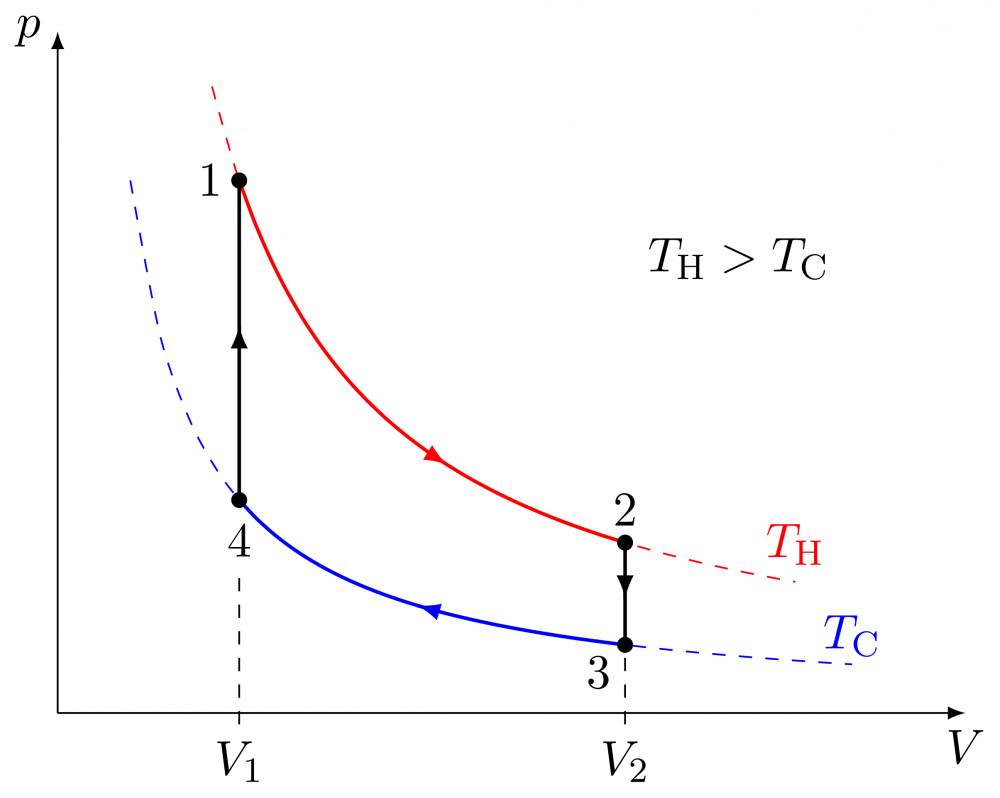
Then let’s look at the Stirling cycle https://en.m.wikipedia.org/wiki/Stirling_cycle Diagram from link 1→2 Isothermal heat addition (expansion). 2→3 Isochoric heat removal (constant volume). 3→4 Isothermal heat removal (compression). 4→1 Isochoric heat addition (constant volume). https://en.m.wikipedia.org/wiki/Stirling_cycle#/media/File%3AStirling_cycle_pV.svg Heat in, heat out
-
Jupiter is ~5.2 astronomical units from the sun, so it gets (1/5.2)^2 as much energy per unit area, or ~1/27, which is about 3.7%
-
It’s the “Period” which implies that there is nothing else. It means that no heat is transferred out, which is contrary to mainstream physics. The notion that the work is solely driven by the heat in and work out, and that these are equal, is not mainstream physics. “Work goes out, the temperature goes down. Period.” is not widely accepted. There are examples of work without heat flow - adiabatic compression and adiabatic expansion against pressure or some other impediment, like a spring. But these are only individual steps - they are not the entirety of a heat engine. There’s no heat in or out. All there is is the work being done, which changes the internal energy. https://en.m.wikipedia.org/wiki/Adiabatic_process#Adiabatic_free_expansion_of_a_gas
-
A drop in temperature might be the result of heat flow, and might be due to work being done. You did not provide enough detail to say further. But since heat flow could be present, concluding there is no heat flow is incorrect. I guess that’s the problem in a nutshell: You don’t see how my comments apply to what you said. But what you said wasn’t true. To the point of the thread topic: Heat isn’t conserved, but it isn’t destroyed, either. In mechanics, kinetic energy isn’t a generally conserved quantity, either, but it can change into other forms. It isn’t “destroyed” - such a description would be misleading. Ultimately you want to be able to quantify effects, and that means using equations. Equalities are generally the most useful. “heat is destroyed” doesn’t lend itself to solving an equation.
-
Heat flow does not create a difference in temperature. If there is no temperature difference, there is no heat flow. Q=0, since Q depends on a temperature difference Not true. If energy is transferred owing to a temperature difference between objects, it’s heat. Work is energy transfer that doesn’t depend on a temperature difference. You don’t get to change these definitions. Also, do not confuse a temperature change in time with a temperature difference between objects.
-
Reminder that status updates aren’t there for subjects that should be discussed in the forums.
-
This is a semantics argument, about phrasing. One reason why we use equations to make things clearer. But since language us still used, we try and define things as clearly as possible. Yes, heat is a transfer of energy between objects (or systems) owing to a temperature difference (sometimes called heat transfer). That distinguishes it from work, which is other energy transfer. There is also energy content. Sloppy use of terminology often leads to confusion Energy is conserved. Ei = Ef There are different forms of energy, so each of those might be broken down, depending on the details of a problem (potential, kinetic, thermal, etc) Energy can be transferred in our out of systems, so that’s another thing that must be accounted for, like balancing one’s checkbook. Ei = Ef + W for example It’s important to define the boundary of a system when applying these equations. In the context of the first law, energy is not destroyed. Heat is not destroyed. The energy goes somewhere. Argument to the contrary is semantics, and trying to skirt the issue by stretching the narrow definitions we have in physics. When in doubt, refer to the equations.
-
! Moderator Note The problem is that you do not provide evidence in support of your theories. You’ve had multiple threads where you tap-danced around the inquiries of others. Your Carnot thread went to the 17th page - there was nothing abrupt about it. You’ve had other multi-page threads. Where do you respond in support of your “theories”? At this point, you do not have a place here. You had ample opportunity, and you’ve used up the goodwill extended to you. You can ask questions and get answers about mainstream physics. That’s all.
-
! Moderator Note Anything that doesn’t belong elsewhere, and doesn’t violate the rules If you can manage to ask questions and not preach your own version, or bring up extraneous material, the proper place is in classical physics.