-
Posts
1457 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by aommaster
-
so, if i put a seed crystal, will any salt form on the thread???
-
then, when does it combust incompletely if we have all the oxygen we want (from the room) ???? Sorry to bother u, but, i can't really get to grasp with this
-
and what job exactly does the seed crystal do??? Because i tried to put a thread in the solution, and the crystals grew all over the thread!!!
-
OH sorry sorry i didnt count the 4 in fornt of the carbon ok thanx
-
oh ok. By the way, how can i get a slat seed crystal, their sooo TINY
-
yeah, cause, looking at the equation: c4h9oh + 4o2 ------------> 4co2 + 5h2o | 3O2 is needed to correct it
-
shouldnt it be 3 o2 as in ur equation there are 9 on the left and 7 on the right???
-
and thats it
-
lol well no, not really LOL because it can either burn completely or incompletely i want to know how it would burn NATURALLY and to aid that, i want to know how much oxygen for a reaction is that maximum possible at room ixygen levels so that it changes from being a complete to incomplete combustion
-
so is the balanced equation: c4h9oh + 8o2 ------------> 4co2 + 5h20 ?????
-
Well let me explain this scenario: 1. You are given a hydrocarbon 2. You cannot experiment to see whether soot is released 3.You have to find out whether is burn completely or incompletely 4.This is ALL at normal room oxygen levels i think 30% How can u tell whether it burns completely (Water and co2 released) or whether it burns incompletely (water, co2, co and C released)
-
well, honestly to tell you, i havent a clue what you are talking about!!! But all i know, is that the butanol we are using has a formula of C4H9OH P.S i read ur question on the smallest movement that is detected and i have a question, Why do u ask impossible-to-answer questions lol
-
by the way, there is no experimenting !!!!
-
But how could you tell how it would normally combust, as there are two ways it can go
-
i have got these bond energies: Butanol 5580 (total) Water 928 Carbon Dioxide 1210 oxygen 498.3 When i work out the total bond energies on both sides, it shows me that the complete combustion of Butanol is ENDOTHERMIC!!!! Whats wrong with my calculation C4H9OH + 6O2 ----------> 4CO2 + 5H2O 14149.8 11080 See?
-
OK, i think a minor misundstanding. Lets say, you have an alcohol, propanol and it is involved and a combustion. How can u tell, without looking at a balanced formula at room oxygen levels, wether it is imcomplete or complete combustion. You only have the name of the chemical and two options for a formula, complete or incomplete
-
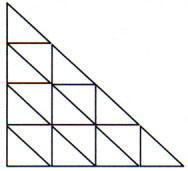
I think youve made a mistake. You only found the number of small triangle, i need you to find EACH AND EVERY triangle. There are bigger trinagle like the whole of the first and second row. lol
-
-
thanx i will One more question, i tried this experiment in advance, when there is slightly more salt in the solution, the salt forms 'snowflakes' and floats above the water, how does that happen?
-
In a normal room with normal levels of oxygen, and without any testing or experimenting, how could you tell if a substance burns with complete or incomplete combustion???
-
oh ok ill try the magnesium ones then thanx
-
Is it possible to grow copper sulphate? And how? Are there any hazards for magnesium sulphate?
-
i need something that i can grow at home
-
If i wanted to grow salt crystals to their original shape (Cuboid, i think), how would i actually do it. I know the basics, like make a saturated solution, but how should i grow the seed crytal and how to obtain it Thanx
-
umm... i don't really have a picture, i was just generalising, i just wanted to know if there was a formula. Can u explain to me exactly how to find the formula so that i could work it out by myself