-
Posts
1710 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by mississippichem
-

IS GRAVITY ONLY A MANIFESTATION OF MAGNETISM?
mississippichem replied to rigney's topic in Speculations
I don't know a whole lot about astronomy; No more than the introductory stellar astronomy course taught me anyway. So I've got a true layman's explanation for you, rigney: Whenever another planet passes between Earth and the sun, we don't get any magnetic shielding effects. Also, whenever objects approach another astronomical body, they don't align themselves to the magnetic field accordingly. We've never seen an asteroid or comet get repelled by the earth is what I'm trying to get at. -
CO isn't a strong enough reducing agent. [math] E^o [/math] for the reaction [ce] Na^{+} + e^{-} -> Na^{0} [/ce] is about -2.71 V relative to the standard hydrogen electrode. One would need a really strong reducing agent as the Na metal that would form will be very reducing itself.
-
Yes. It is in fact the preferred method of mass scale sodium production. They use doping agents to lower the melting temperature though. However, I doubt that anyone does this at the lab scale, liquid sodium chloride is some really nasty stuff.
-
Not sure about celery. But some foods like some lettuces (nothing but un-digestable cellulose) gives a net energy loss because energy is spent consuming it and it contains little to no nutrients that humans can digest. Cows and other grazing animals have no problem with cellulose though. It is their staple. Down with lettuce. Long live Spinach.
-
Helium has been known to form Van der Waals complexes with "soft" metal centers* (usually highly polarizable [math] p^2[/math] or [math]p^4[/math] centers). However magnesium has a very tight full s orbital and has no energetic need for a dative interaction and not much polarizability for Van der Waals Complex formation. *Some of these complexes are metastable at STP but many are only observed by far IR spectroscopy in a super cooled solid argon matrix, as just room temperature thermal energy is enough to cause cleavage of these super weak interactions. There is no heterolytic seperation of ions or homolytic spin "de-pairing" that needs to occur.
-
Not to mention you would need to synthesize them all in under a week or so, because people are constantly discovering or making new compounds. Ever since DFT and synth robots came out, synthetic chemistry is advancing extremely fast; mostly on the organic front, but I'm sure there is a new copper species at least every week. As for copper(II) borate...not really sure either. But I imagine you can find a solvent that it will percipitate in. Then just find a soluble Cu(II) species and a soluble borate species. Stuff like this usually works for simple ionic salts. I think borax is a tetraborate hydrate though, don't know how far you'll get with that but it might work.
-
Yeah, I just think NIN songs are catchy and are well written pop songs (this might be an oxymoron). Trent Reznor worshipers are almost as annoying as Thom York worshipers. Both receive far too much credit and are hailed as musical geniuses but both are really just "pretty good" pop writers. Reznor has great production quality though. I've got respect for that seeing as how he records his own stuff.
-
NIN is awesome. Great highway music.
-

MgBr+ -- Where is the charge at? What is the name of it?
mississippichem replied to Genecks's topic in Inorganic Chemistry
Though the ion only exists fleeting in a Grignard solution, its formal name is bromomagnesium(II). -

MY ACTUAL PERPETUAL MOTION DEVICE...with VIDEO EVIDENCE....
mississippichem replied to Kris K.'s topic in Speculations
So I guess you've purchased a lot of capacitors and you're no longer paying the power bill? -
What full equation? No, the math is easier to read for many of us.
-
Fiiiire on the hill run boys run...(fiddle, fiddle, fiddle)...Devils in the house with the risin' sun...(fiddle, fiddle, fiddle)...
-
Yes an oxidation. But whenever there is an oxidation there must also be a ________. So this is an oxidation-________ reaction.
-
A History of Chemistry: From the Earliest Times till the Present Day by James Campbell Brown: The book was published in 1913 so a lot of concepts that seem revolutionary to the author are elementary to us now. I like it because it gave a nice insight into the collective conscious of chemistry right at the time quantum mechanics ideas were beginning to bounce around. Chemists at the time still hadn't figured out the difference between covalent bonds and ionic interactions so it's interesting to see their mistakes. Be careful though, there are a lot of factual inaccuracies in the book so one must keep in mind that this was written in 1913, before modern spectroscopy, intricate bond theories and full disclosure of Maxwell's dynamics to chemical systems.
-
Nitrate is one of nitrogen's highest oxidation states. Ammonium nitrate is used as an oxidizer in some explosives. That's a pretty strong hint. Also, have you ever heard the term "reducing sugar"?
-
It would be interesting to see a study. I don't have a pHD, but I intend to start one soon. I've noticed that the more stuff I learn the more cynical I get .
-
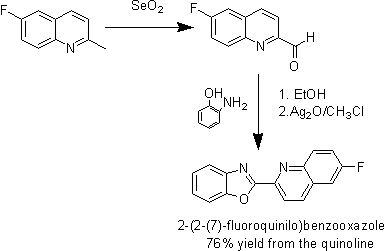
Some of my undergraduate work from last year. I worked organic synthesis in a coordination chemistry lab. I like this reaction because it is high yielding and gives nice yellow crystals. The [ce]SeO_2[/ce] oxidation was my idea, as I had seen similar Ar-methyl oxidations in the literature. Interestingly enough, we actually got a small amount of over-oxidation to the carboxylic acid which technically shouldn't happen. 13C NMR in CDCl_3 showed a peak at 193.579 which can only be accounted for by a small amount of the carboxylic acid. The thiazole and imidazole were also prepared with 2-aminothiophenol and ortho-diaminobenzene respectively instead of 2-aminophenol. I have read in literature that the final ring closing can also be accomplished with [ce] O_2 [/ce] gas bubled through xylene. We decided to to use the silver(I) oxide instead though. 1H NMR (CDCl_3): 1.20(s), 1.65(s), 7.19(s), 7.61(m), 7.81(m), 8.31(d), 8.71(d) IR: 401, 628, 703, 738, 1038, 1076, 1438, 1451 The thiazole analogue was later chosen to be reacted with the ruthenium center because the oxazole was too labile at the desired pH. I should add that the whole scheme scales up very well. The first time I ran it I made about half a gram. The next time I made 40 grams and got a very similar yield.
-
By not having dimensionally correct equations you are essentially claiming that 1 apple + 1 orange = 1 banana. Equations aren't just there to look pretty or complicated. They actually have to make logical sense. Physics isn't about redefining logic. Physics uses math, experiments, and logic to draw conclusions about patterns and events that occur in nature. One week of a high school calculus course would show you that your "definition" is nonsensical.
-
Whenever people come to my door asking if they can share their beliefs, I always say "Yes, as long as I can share mine with you". It usually ends up being a polite yet wonderfully tense conversation once they realize I'm not budging. They get a bit uneasy though when I break out the RNA world hypothesis or start a mitochondrial DNA rant. I grill the hell out of them usually, but I try to keep a smile and a helpful attitude. If they invite me to church, I invite them to a chemistry or physics seminar at my school. I tell them that is my place of worship (and it is ). Almost every event where ideas are exchanged in a polite and constructive manner is good though; what harm can come from a friendly debate? I think none. Turn in your bible to the book of statistical thermodynamics: -Thou shalt conserve thy energy -Thou shalt conserve thy mass. Yea except for thine holy mass defect in whence case thine energy shalt still be conserved...
-

My questions upon time discrepencies are these....
mississippichem replied to seasnake's topic in Relativity
You must have finished school before they started offering the magical vacuum dynamics course. You really missed out. -

I'm writing a scientific paper for a Journal
mississippichem replied to Bloke of the forest's topic in Amateur Science
Thanks, I should have pointed that out. The best way to compare impact factors is within a given field for sure. By the way, I've never really been a fan of "Nature". I've always felt that "Nature" wanted to be an interdisciplinary journal but secretly is a biology journal. Notice that every now and then they tag on some cool physics or physical chem just to stay relevant to us super nerds. -
You can find any publicly traded company's balance sheet, retained earnings statement, statement of cash flows, or income statement on "google finance". Be sure to read the auditor's notes.