-
Posts
630 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by wolfson
-
Molar mass of Ba(OH)2 --- 137.3 + (17 x 2) =171.3 Number of moles of Ba(OH)2 in 0.212g =0.0012 moles Molarity = Number of mole (n) / volume of solution M = 0.0012 / 0.25 = 0.0048 Molarity [OH-] = 1.0e-14 (e=x10) / 0.0048 = 2.08e-12 mol dm^3 (1e-14 is the water constant Kw). In basic solns, the [H+] < [OH-] \ [H+] < 1 X 10-7 M
-
I know what it is, but without additional text it can give the wrong impression. Yes your right, typical american sites. Well one day ill get round to sorting it out, budullewraagh should also learn basic chemistry and non of this would have happened, oh well maybe we can teach him the English chemistry here
-
another refference that you may wish to look up is the Pauling scale, that also clearly states that Li = 1.0 and K =0.8 the lower the number, the more electro-positive the element is Thats totally correct (hands clap) good reference YT
-
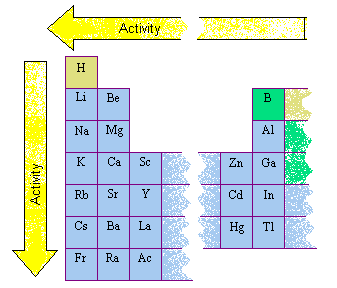
Its a DEX diagram, the arrow is applied for metals only, it shows the rule thats all you need to know.
-
1. I have looked at most of the sites (america) and NOT ONE says that Li is more active than K, istly Active means Reactive. 2ndly these sites just put the active elements in there groups, it may look as if Li is more active than K, but that is just because Li is above K in the periodic table of elements, YOU MUST learn that reactivity increases as you go down g1. I have attached a picture explaining it to you, FROM an AMERICAN site.
-
Budullewraagh GIVE it UP, you are wrong, its not england thats wrong its you, AGAIN POTASSIUM is MORE ACTIVE/REACTIVE than LITHIUM
-
Kinetic energy increase, therefore potential energy decreases.
-
As a chemist i will tell you straight POTASSIUM is MORE REACTIVE than LITHIUM. And saying england is wrong is far far from the truth.
-
For KClO3 look at: http://www.fryingcolors.com/kclo3.html
-
Heterogeneous liquids are opaque because the fine particles suspended in the liquid scatter light. A clear liquid will be homogeneous.
-
If you want to mass produce any product you must understand BASIC chemistry, if you increase the pressure and temperature of Cu and H2SO4 you will get your CuSO4, add a catalyst to maximise the inital rate.
-
Limewater = saturated calcium hydroxide solution. HCL = acidic aqueous solution of hydrogen chloride * solution = homogeneous mixture of two or more substances.
-
Communications 2.6 megabits per second Storage 316.4 kilobytes per second 1MB file download 3.2 seconds Subjective rating Great
-
Add H2So4 (sulphuric acid) to copper Equation: Cu + H2SO4 > CuSO4 + H2 So 1 ton of Cu and 1 ton of H2SO4 = 1 ton of CuSO4.
-
I so thought the inital question used a group 2 metal not a group one, for the calculation dont use the molar mass of Na(OH)2 use NaOH, so the pH will not have a 2 x molarity, i though it was 2+(OH)2 my mistake have to look more carefully
-
Well the new solution reaches its max/perfect equilibrium at -22oC, so any where above that it would not be totally in the equilibrium. So if the temperature outside is -5 the ice would melt, but it is was -24 outside it would not melt. Attach is basicly reaching the equilibrium, so yes melt/dissolve before the max -22oC.
-
Ah yes acids and bases where were we?
-
What was the actual topic?
-
http://search.yahoo.com/search?p=chemical+dynamic+equilibrium&ei=UTF-8&fr=fp-tab-web-t&cop=mss&tab= look at how many there is READ them and LEARN.
-
One isotope of Francium (223Fr) has a half life of 21.80 minutes, made by alpha decay, and is normally studied in laser atom traps. Astatine can be produced by bombarding bismuth with energetic alpha particles to obtain the relatively long-lived 209-211At, which can be distilled from the target by heating in air.
-
Formation of poly(ethene), when catalysed and heated under pressure, alkenes link together when the double bond opens. The spare bonds are used to join up the molecules. The original small molecule is called the monomer and the long molecule is called the polymer, which is the sort of molecule most plastics consist of. The polymer is now a saturated molecule but has the same C:H ratio as the original alkene. So lots of small molecules join up to form a big long molecule in a process called addition polymerisation and the polymers are named as poly(name of original alkene).
-
Well 1 mole = 6.022e23 atoms so: Mass (Mr) of Fr (1 mole) = 223g's So mass = Mr / n = 223 / 6.022e23 = 3.70e-22 grammes per atom. 17 x 3.70e-22 = 0.0000000000000629 g So no
-
Id more than likley kill myself in the process of my fireworks display.
-
Oh a superacid plus caesium, fireworks display