John Cuthber
Resident Experts-
Posts
18413 -
Joined
-
Last visited
-
Days Won
52
Content Type
Profiles
Forums
Events
Everything posted by John Cuthber
-
Would you like another guess? https://en.wikipedia.org/wiki/Potassium_bitartrate#History If a chemistry expert tells you something has a low solubility, then they are unlikely to be talking about something that's very soluble.
-
One possible problem you will face is that potassium hydrogen tartrate isn't very soluble. Another is that the process of dissolving sodium hydroxide in water can be violent, even without acids present. And your KOH is probably only about 85% pure- the rest is mainly water.
-
The rochelle salt lattice only really works with equal numbers of both cations (you can substitute some NH4 or Rb in place of K if you like).
-
Get well soon. It's the latent heat of evaporation.
-
You can do chemistry this way but it is horribly inefficient. It's difficult to maker perbromates (and amusing to look at old books "explaining" why they are impossible) One way to do it was to make a selenate with the right isotope of selenium, and wait for it to decay. https://en.wikipedia.org/wiki/Perbromate#Synthesis
-

Twenty-five years since Y2K, lord what a bruhaha.
John Cuthber replied to gawdzillasama's topic in The Lounge
It's a bit like that thing where they put lots of effort into eliminating smallpox. Total waste of time because you just don't see it anymore. -
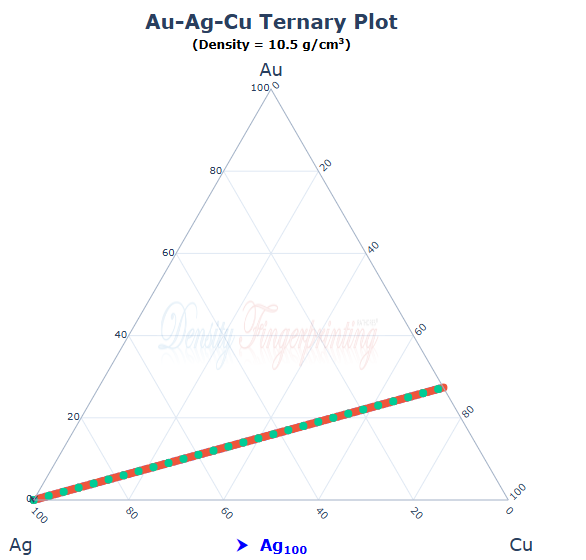
It's the actual density of silver What does that mean? Is it something your model does? Do you realise that your model does not affect the density or composition of a piece of metal? How can it identify pure silver? It can't distinguish it from a silver/ copper/ gold alloy with the same density, and there are essentially an infinite series of those.
-
Are you deliberately missing the point? The actual density of silver is 10.49 You can invent any alloy you like with that density, and I will tell you that you have got it wrong because the real alloy it has more silver (Or less) than you suggest. Changing the silver content does not ( to a first order approximation) change the density at all. If you choose to be a bit more precise then adding silver changes the density slightly. But you can always get it back to exactly 10.49 by adding either copper or gold. So, whatever composition you suggest, I can say you are wrong. Your method simply can not work.
-
The device rejoices in the name of an earth inductor. https://geomag.nrcan.gc.ca/lab/vm/inductor-en.php
-
In the particular case of the CuAuAg system, I think the colour would give you a hint. If you measured the reflectance spectrum carefully, it would probably be sufficient. The electrical conductivity might also work, or the hardness, or the speed of sound, or the melting point or magnetic susceptibility or the electrode potential or melting point. But the point remains; you need something to tell you where on that line you are. If you are in the UK and there's a point on the line corresponding to 9 (or 18) carat gold then that's a fair bet, but no proof. But in the USA they seem to prefer 10 carat (as far as I can tell).
-
No, it's not. If you think I'm wrong, it's up to you to prove it.
-
The clever bit about those is not the choice of material so much as the big gaps. That means they are less troubled by expansion. They don't buckle and warp because, for the most part, they can just expand a bit. They use cast iron because it's strong enough, has a high enough melting point, and it's cheap. A sheet of cast iron would only be slightly better than a sheet of glass.
-
Repeatedly taking away a debt is the same as repeatedly adding a credit.
-
Copper is less dense than silver. Gold is more dense than silver. Therefore there is a binary alloy of copper and gold which has exactly the same density as silver. We can call it "match" because it has a density that matches that of silver. An alloy of mainly copper with a little gold will be slightly more dense than copper. We can refer to this alloy as "light" and similarly we can have an alloy, which we can call "heavy" made from mainly gold with a little copper. If we start with pure silver we can add a small amount of heavy which will raise the density, and then we can add light to reduce the density. The overall effect will be to produce an alloy which has the same density as silver and which is a ternary alloy. We can repeat that process and get a second alloy- again containing all 3 metals and which has exactly the same density as silver. It will have a different composition from the first one. And we can , add further amounts of light and compensating amounts of heavy until we get arbitrarily close to "match". All these alloys have the same density as silver. How does your method distinguish among them? (The option of extending this to have an alloy with, for example, a density half way between that of gold and silver or whatever also exists)
-
I think we should tell everyone that. And then the Reps will get complacent and teh Dems will get determined. Anyone remember how the UK's Brexit referendum played out? We all knew that it would fail...