John Cuthber
Resident Experts-
Posts
18386 -
Joined
-
Last visited
-
Days Won
51
Content Type
Profiles
Forums
Events
Everything posted by John Cuthber
-
And, once again... A well might contain excess CO2, but it's not there because it "fell in" from the rest of the atmosphere; it's there because it was produced there by bacterial action. So there's no disagreement with the document. I disagree with you that it has any relevance to the OP. Feel free to explain the link between the location of CO2 from fossil fuels, and CO2 generated in wells and taking time to diffuse out. "There is experiment, that probably anybody who has access to dry ice, tried at least once in life. Paper boats, balloons or bubbles, floating in empty aquarium." Yep, But why do you think you need the dry ice? According to your idea, all I need to do is leave the tank open and CO2 will build up in it by stratifying out of the air. The rest of us know that , since we can take a bath without asphyxiating, that simply doesn't happen Or, you could stop digging.
-

What happened to educational TV channels?
John Cuthber replied to Hans de Vries's topic in The Lounge
Did any of them cover the use of the apostrophe? -
There isn't a lot of oxalic acid in potatoes: Estimates vary http://www.childrensdayton.org/cms/resource_library/nephrology_files/5f5dec8807c77c52/lithiasis__oxalate_and_diet.pdf http://www.wakehealth.edu/Urology/Kidney-Stones/Oxalate-Content-of-Foods.htm and it's not particularly likely to act as an anti misting agent. it's not on that diagram because there's just 0.006% to 0.03% or so.
-

How did Newton came up with the idea of prism experiment ?
John Cuthber replied to Chriss's topic in Classical Physics
The first person to observe the effect would have been someone who had a piece of ice and some sunlight. It probably happened before anyone had invented writing. How could it be recorded somewhere? -

Should we hide the identities of presidential runners?
John Cuthber replied to silverghoul1's topic in Politics
Wrong on all counts. -
I told you it was amusing. For example the reason why this chunk... "You said you would try drying it (but the quickest drying is done by heating substance! To evaporate water). Drying at room temperature, of larger amount of juice, would take days if not weeks. Unless you use yet another substances that reacts with water (f.e.desiccants)."... is wrong, is called freeze drying. Re "the main question in PM was: how are you going to identify unknown compound in unknown solution (mixture of unknown compounds) using XIX century knowledge " Nope, if you look really closely you will see that the OP didn't mention "XIX century knowledge"- that's just something you made up. Re. "ps. In 2015, I was doing 50 distillations per month.. >400 in whole year. Don't believe? 18-28 February 2015 http://www.sciencefo...lation-results/ " It's not a question of whether I believe you or not is it? It's an issue of whether or not it matters. BTW, you might want to look at this https://en.wikipedia.org/wiki/False_precision I hope Strange won't object to me "pinching" a reference he used in another thread. https://en.wikipedia.org/wiki/Law_of_holes
-
"You clearly misunderstood my example about the hole in the ground." No I more or less ignored it because it was irrelevant. But it id dhow that you understand that diffusion happens slowly. And you did, on the other hand, say that it happens quickly. "I said, and I meant, that the Earth's atmosphere ie stratified." Yes, you said it. Yes you meant it No it isn't- at least not this side of space, which means the overwhelming bulk of it is not stratified. Saying it again is no more helpful than strangling the English language with things like " for all that working off of the Sunday roast." "And that that statification is due to the varying pull of gravity on molecules of diffeent molecular weight." As the data you cited show, that stratification is non- existent (except in the vacuum of space)- and I showed you the calculations that explain why; even without wind and convection currents, it would be pretty well mixed. "Why do you think gas detectors in buildings are mounted on the ceiling not the floor?" At least in part, because they don't get covered up if they are up there. But you seem to have missed the point about slow (the time-scale over which the atmosphere has formed) and fast (the time scale over which a room fills with gas). "Now note layer 5 in particular. Mostly hydrogen." Has approximately sod all in it because it's a vacuum. It has nothing to do with the OPs question. Now, please get back to us after you have come to terms with three simple facts. For the overwhelming bulk of the atmosphere- by weight, or number of molecules- the composition is pretty close to the same at at ground level. The atmosphere has had plenty of time to mix. You were wrong. In the mean time, I suggest you take Strange's advice and stop digging.
-
The exchange of PMs between me and Sensei about this is, in my opinion, quite amusing.
-

Should we hide the identities of presidential runners?
John Cuthber replied to silverghoul1's topic in Politics
Well, if the alternative is Donald Trump... Given how untrustworthy a typical politician is, that might not be a bad thing. It would force people to actually think about what the candidate is saying, which would be a start. -

Verbal tics
John Cuthber replied to petrushka.googol's topic in Anatomy, Physiology and Neuroscience
There are probably links of a sort- if you are nervous you are more likely to "err and umm", and you are likely to have a raised heart rate. But I doubt there's any strong causal relationship. -

New Electromagnetism: An improved model of Electromagnetism
John Cuthber replied to ForcefulLorentz's topic in Speculations
" Firstly, in order for us to make a proper argument by authority (not logically fallacious) we need to confer with said authority." No we don't. It is sufficient that the authority has had an opportunity to judge the idea. they have. It didn't pass muster. No conference is required. "This decision is not a scientific decision, but an editorial one. It is editorial because it regards how the submission relates to the journal as a whole as opposed to science itself. " That's an unsupported assertion. The only scientific editor I know is also a scientist and she sometimes has to reject papers because they simply are nonsense. The way that put they- to avoid causing more offence than is needed- is "this isn't the sort of thing we publish. "Secondly, you're essentially making an appeal to probability anyway." Appeals to authority always are. Even Atlas shrugs. -

Are alternative fuels pretty much done for?
John Cuthber replied to Elite Engineer's topic in Engineering
If trashing the environment in the pursuit of profit was a one-off you would have a point but as I asked- when did it work in the past? in any event, there are less fragile places you can extract energy. -
OK, so lets sum up I said 1 check that it actually works 2 Don't assume that the active ingredient is heat stable and volatile. Whereas you assumed that the best thing to do was make mashed potato. What would have been different in the 17th or 18th C? Would you like to revisit these ideas? "I am just suggesting that research can be done by yourself. It's much more entertaining, and brain developing, than just looking up somebody else work and read.. It's like solving puzzle by yourself, versus letting somebody else do it for you.."
-
Meanwhile, back at the topic; I wonder if sugars and /or saponins are the active ingredient.
-
"Dumb" and "troll" are a long way from being mutually exclusive.
-
Indeed, and you would then find that potatoes do contain a little oxalic acid - which is used (at much higher concentrations) as a cleaning agent. However, it's not particularly likely that it would work to stop things misting up. So I'm leaning towards the OP's opinion (perhaps just a guess) that there's some sort of surfactant present. If it's the oxalic acid that makes a difference (TBH I doubt it but...) you should add spinach or a rocket salad.
-
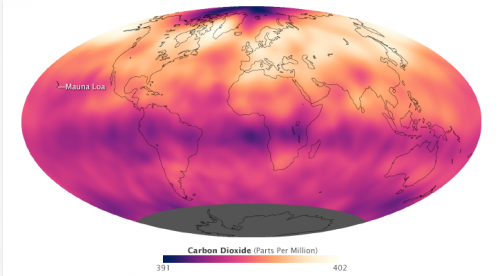
"Two responders here have chosen to limit the atmosphere quite arbitrarily." Along with everyone else. If you don't then you end up having to include the whole of the universe and that's plain silly. Practically none of the earth's atmosphere is above 100 KM so it's perfectly sensible to ignore it. And you are now starting to argue against yourself. This question "Consider a shaft in the ground 2 metres square in section, 5 metres deep and open to the atmosphere. However the the atmosphere in the shaft comprises over 20% carbon dioxide. How long will it take for the atmosphere to equalise with the lower % in the normal atmosphere above?" is a question about diffusion. And diffusion is the process that spreads the gas horizontally away from cities etc. But, as you say "horizontal distribution is fairly even since the mixing times for a gasesous atmosphere is measured in days." So, you are saying that horizontal diffusion is fast, but horizontal diffusion is slow. So, having discarded the "how long" question because you answered it (albeit twice, but with different spin). you then need to look at the equilibrium concentration. (because the atmosphere has been here a long time - the eqm with the ocean is a different, slower matter). The concentration at equilibrium will pretty much follow a Boltzmann distribution. Molecules that are higher will have a higher gravitational energy . The relative populations will depend on the energy differences and on the temperature. (This is true whether you "challenge" it or not- the atmosphere doesn't care about your opinion). OK consider a molecule of oxygen It's mass is about 5.356 x 10-26 kg. And, if it gains an extra metre in height the energy change (Mgh) is about 9.8*1* 5.356 x 10-26 kg. 5.26x10-25 J Now the kinetic energy (up and down) of that molecule is roughly the product of the temperature and Boltzmann's constant. (1.380 × 10-23 J/K ) Say it's freezing cold just to define the temperature as about 273K. That gives the molecule a thermal energy of about 3.77 *10-21 J So the kinetic energy in each direction is roughly 7162 times more than the gravitational energy of rising my 1 metre. There are 3 dimensions the molecule can move in 21486 times more kinetic energy than garvitational So the ratio of the populations of those two states is about e^ 1/41486 which is something like 1.000046. (that's an estimate of how much higher the pressure is at ground level,compared to 1 metre further up. Now, if we do the same thing with CO2 molecule we get a KE to thermal energy ratio of 21486 *32/44= 15626 (the thermal energies are the same thanks to the equipartition principle and the CO2 molecules gravitational energy is 44/32 times higher because it's heavier) And the corresponding ratio of populations is 1.000064 So the enrichment is something like 1.000046/1.000064 per metre The expected change in concentration ratio of CO2 and oxygen is 1.000018 per metre Something like 1.8% for a kilometre. And remember, that's an upper bound. Any winds or air currents etc will cause it to mix better than that. So it's no great shock to most of us that the figures show that, up to the edge of space, the CO2 levels are pretty consistent with altitude. Now you may remember that I posted a link to a picture of the worldwide variation with location. Here's a picture One interesting thing on that map is the scale. It goes from 391 to 402 ppm. That's about 397=/- 1.4% The horizontal variation is about 1.5% And, according to the page cited by studiot, the variation of practically all the atmosphere with height is less than 1 % So, the following statement he made "The the gaseous makeup of the atmosphere is stratified, like the settling sediment in water with the greatest concentration of carbon dioxide at the Earth;s surface and the percentage of lighter gases increasing with altitude. ... Horizontal distribution is fairly even since the mixing times for a gasesous atmosphere is measured in days." "needs revision" because the stratification is rather smaller than the horizontal variation.
-
Yes it's so. Why have you asked me to explain the figures there? We were talking about CO2 and there are no data on that page for CO2 Also, did you read my post? Did you understand it? Why do you seem not to realise the importance of this bit? "It doesn't usually vary much with altitude- at least not at altitudes where you could still breathe." It's fairly easy to explain the near constant composition- all the way up to the beginning of "space". The thermal energy of gas molecules is vastly greater than the gravitational potential energy. in essence they are moving too fast to "settle out". You do realise that almost all of the earth's atmosphere is below 100 Km don't you? The vast majority of molecules are in parts of the atmosphere where the composition is nearly constant (apart from big changes in water vapour).
-
"Did you try smearing potatoes on the windscreen " No I didn't, but I did say "A really good scientist would start out with a potato and a windscreen..." Winter wash fluid's major requirement is that it doesn't freeze- hence the alcohols. However that's not the question raised by the OP " Never tried as defogger for my scuba mask. Would it work ?" Now, not many people's scuba masks are below freezing so why bother with the properties of a winter washer fluid? Is it because you were hoping to make me look bad? Nice try. "Receiving water from experimental distillation is also information for scientist:" Well, yes, the fact that potato has a lot of water in it is information. but I rather suspect that it's information already known to most scietists. "I don't see how that would help to determine molecular structure of compound?" You have missed something; there is only one report that the substance exists. It could be that modern spuds- perhaps a different strain or whatever- might simply not work. So you could be making mashed potato for no reason at all. The first step is to actually check that the effect is repeatable with the potatoes available to you. It might also be amusing to see if potatoes still work when they are boiled. if not then we could conjecture that the material responsible is not heat stable. And that would also invalidate your suggested experiment. Seriously, what is the distilled extract of spuds going to tell you in this case? As far as I can see this suggestion of yours; "True scientist would blend potatoes in blender, get juice, fill distillation flask, perform distillation, and try to gather larger amount of product(s) (for various boiling temperatures, refining)." is pointless- not least because it doesn't actually involve windscreens.
-
The stratification is small. Generally too small to consider.
-
In general, it's mixed into the atmosphere as a whole.However there's a slightly uneven distribution because, for example, the Northern hemisphere produces more than the Southern one. Locally you get "clouds" of it above major cities and countries with denser populations. It doesn't usually vary much with altitude- at least not at altitudes where you could still breathe. Here's a map http://earthobservatory.nasa.gov/IOTD/view.php?id=82142
-
I have the sort of friends who would say "Why didn't you just ask me to reduce the number mod 9" when I got to step 2. (Well, not all my friends would do that. some would complain that it doesn't work- because they do all their arithmetic in octal, hex or binary.)
-

Are alternative fuels pretty much done for?
John Cuthber replied to Elite Engineer's topic in Engineering
can you show me an example of where that has worked in the past? -
The real problem you face there is that sulphuric acid reacts with sulphur under those conditions. 2 H2SO4 + S---> 3 SO2 + 2 H2O
-
Unless you actually want something else. In that case a compulsion to say vodka would be a problem.