-
Posts
1704 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by Lance
-
Yes. It’s the cheap and simple one. It’s just a tank, valve, and nozzle. I used a 24V power supply. I don’t even remember where it came from. It’s current limited at 4A. It would be much faster if it could supply a higher current. A higher voltage will allow more current to flow, but a care batteries should work fine. I took two pencils and sharpened both ends of both pencils. I then hooked up my power supply to them one at a time and waited for the wood to burn off. I think that this method is too impure though because they disintegrate quickly. I’m going to go the hardware store today and pickup a lantern battery and use the thick carbon rods in that instead. Sodium has a lower melting point then Sodium chloride so it mixes with the salt. Not that I know of. I couldn’t find anything. As far as I know I'm a pioneer in the armature science community. If you find anything please let me know. I believe so but I would avoid the word "all" and replace it with most. I have only tried it with KCl and NaCl because they are so similar. Of course I’m still having a problem with getting unwanted yields though. I have tried it with impure graphite, iron, aluminum, and some unknown metal and all have given the same results. I’m thinking the problem may be that the current was not applied ling enough and its still mostly salt. But it should still release SOME bubbles of hydrogen in water. Any input?
-
What's wrong with fast food restaurants, neon signs, and chain restaurants?
-
I thought we shared a room with the physics forums...
-
And I had to stop because the lead broke: I still don’t understand why the sodium/potassium doesn’t create hydrogen when I put it in water... any ideas?
-
I used KCl this time because it doesn't explode and throw hot pieces of salt everywhere. The melting point is only slightly lower. I did this on a brick. Edit: if the pictures dont show up then hit refresh
-
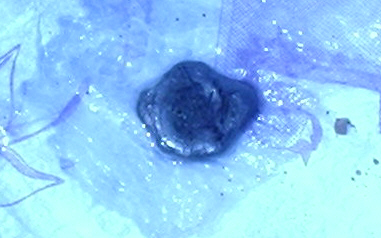
Ok well I successfully melted the NaCl with a plumber’s torch. The salt glows red when it’s fully melted. I used 2 graphite pencil leads for electrodes. I charged them to 24v and the mixture bubbled and swirled a darker red. This also lowered the melting point as it mixed with the salt. When it cooled it was a uniform metallic black. Whatever the product is it doesn’t look like sodium and it dissolved in water without making hydrogen. I’m thinking I didn’t apply the current ling enough and the product was still mostly salt with just a little bit of sodium. When the current was on I did get a big whiff of chlorine though. I definitely wouldn’t recommend doing this inside like I did. Anyway, a picture of the product it below on a napkin covered in mineral oil. Well I suppose you guys saying you have never done it just made it more of a challenge. If you want to try it your self plumber’s torches cost about $12 including a 13oz can of propane. Edit: I’ll melt some more and take some pictures for those that are skeptical.
-
Hmmm... Well imagine that. I HAVE successfully melted it with a blow torch but the problem is the valve for the blowtorch has been converted to the valve for my burner so I can no longer use the torch. I don’t see why the burner would not melt it. Also, fuel is dirt cheap here. The 13oz bottles of propane I'm using cost $3 each. Mapp gas is similarly priced.
-
I have been trying to make Sodium metal by electrolysis of sodium chloride but I just can’t seem to get the salt to melt. I have been using a metal container over a Bunsen burner which should be hot enough to melt it. The melting point of NaCl is 800C. The underside of the container even turns red. Would this work better with a class beaker to keep the heat from sinking away? What temperature can the borosilicate beakers withstand? I used "sea salt" because that’s all I had. Could this contribute to the problem? I figured impurities would just make the crystal structure weaker lowering the melting point. Also As its heating the salt grains seem to explode as if there was pressurized gas trapped in them like pop rocks. If I ever do get it melted would adding KCl lower the melting point of the mixture?
-
24VDC though a 120VAC bulb will still make the filament glow and create lots of heat. 12VDC makes the filament glow very faintly. I doubt 12VAC would make much of a difference. At lower voltages it’s just not drawing enough current to really do anything. You need to buy a bulb rated at the voltage you plan on using.
-

oh please give me advice on sulfur with black powder
Lance replied to a topic in Inorganic Chemistry
Isn't KNO3 and sugar pretty safe/effective? -
You would probably get a better reading using the amp meter. As long as you do it for brief periods you shouldn’t need a resistor for AA and AAA batteries.
-
Here somebody asked a similar question.
-
Yes you would be fine. The current would travel through the skin of the suit because it is more conductive than your body. However it would also act as a lightning rod ensuring that you get struck. I don’t think its really about the faraday cage though. Its more the skin affect.
-
Because if we have a gray line its very easy for it to shift in one direction without warning. If a partial birth abortion is ok then it’s not unlikely that killing babies one hour after birth is wrong. And if that’s not wrong what is? It’s not about having in-betweens it’s about drawing a line somewhere.
-
Because it's NOT her body. Then I suppose the father should have the right to make the woman have an abortion? To veto the mothers wish for an abortion?
-
Well what kind of movie would it be if nothing wrong ever happens? It can feature will smith enjoying modern technology.
-
I really liked the movie. [hides in corner]
-
yes, but unless you have huge amounts of power its a bit slow.
-
Be sure to shoot him after you ask him, not the other way around.
-
I think you can get used text books on half.com. Amazon.com also has new and used text books.
-
It was a movie about golfing.... golf-ing. I read that will smith was supposed to be neo on the matrix but he turned it down for Bagger Vance. Bummer.
-
I didn’t read your links but a while ago I read that they publicly denied having anything to do with Christianity. Overnight all the Christian bookstores took the album off the shelf. Maybe something has changed sense then.