-
Posts
4586 -
Joined
-
Last visited
-
Days Won
12
Content Type
Profiles
Forums
Events
Everything posted by hypervalent_iodine
-
Can you show your attempts?
-
I am not a believer in any sort of deity (I was raised by two rather staunch atheists), but I have to ask why on earth one's religious affiliation (or lack thereof) should automatically preclude you from associating with them or accepting their advice? What sort of advice? Why would you not accept it if it appears to be well-reasoned and rational?
-
Samatha Priss has been banned as yet another sock puppet of the above. Jojowasaman, Hilary, Intothedarkagain, and Jojo_anderson have all been banned as sock puppets of one another, as well as being spammers.
-
What have you done so far / where are you stuck?
-
It’s just basic kinetics. Reactions occur when molecules collide at particular trajectories and with sufficient energy. If you have more molecules of a reactant around, you are more likely to get collisions more frequently and thus, the reaction occurs faster.
-
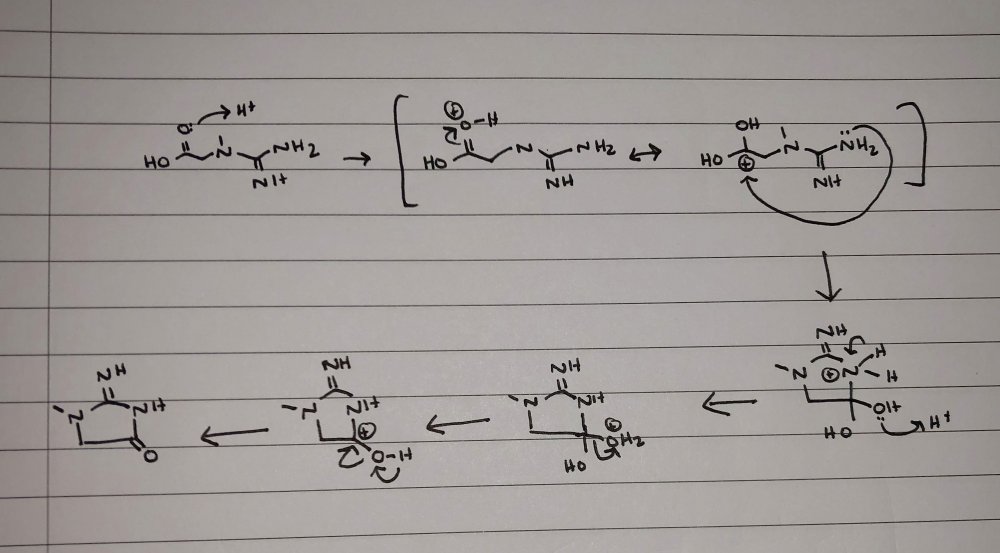
The second step you have drawn out is incorrect. It should look like this: As to why it occurs faster, think about what happens to the carbonyl carbon when the oxygen is attatched to when it becomes protonated. There are two resonance structures of the intermediate that forms, and it has a very big impact on the carbonyl carbon’s reactivity. The H+ you’ll notice is regenerated in the course of the reaction, which makes it a catalyst and is why we say it doesn’t formally participate in the reaction (since it isn’t consumed).
-
! Moderator Note You already have a thread on this.
-
What do you mean by work?
-
Yes, although it is worth noting that a hydride is, as far as I understand, more reactive (and thus less stable) than H+. You could not, for example, have something like NaH just sitting out in the atmosphere without it being protected in some way by paraffin or some such. As I said, I believe this due to electrostatic repulsion of the two electrons, and possibly something to do with nuclear charge. Anyway, point being that as you and I have noted, hydrogen has the capacity to act as either a Group 1 or 7 element to some extent, and is kind of in its own special category within the periodic table.
-
Technically, hydrogen can behave as either a halogen or an alkali metal, though it fits neither category completely and tends to be more of the latter. I would say one of main differences, and probably the most important one, between it and the halogens is in the fact that hydrogen only has an incomplete s orbital. This means that in its ground electronic configuration it only has 1 valence electron. Halogens have incomplete p orbitals, and have 7 valence electrons. A halogen is much more able to grab one single electron to fill its outter valence orbitals than it is to lose 7. Hydrogen, having only one valence electron, can go either way, but prefers to lose one. Couldn’t tell you why losing one is more energetically favoured than gaining one, though I guess it could be due to an extra electron resulting in additional repulsive forces (or maybe not, I’m no physicist).
-
Is this homework? Could you share your attempts at this question and where you are stuck exactly?
-
Much of it comes from ~2015, when you made posts like this: Also worth noting that you have been given good advice on this previously.
-
! Moderator Note (Please do not derail this thread by responding to this modnote; feel free to ask any related questions in another thread or via PM.)
-

Am I losing my mind ???
hypervalent_iodine replied to MigL's topic in Suggestions, Comments and Support
We don’t delete posts. We do hide them. I’d rather not give RC the air time, personally. -
RenaissanceChemist has been banned as a result of being a likely sockpuppet and a definite pest.
-

Am I losing my mind ???
hypervalent_iodine replied to MigL's topic in Suggestions, Comments and Support
It was little more than a poorly veiled attempt to snipe at / about swansont, and clearly not posted for the purposes of genuine discussion. Such threads do not belong on this forum. -

Am I losing my mind ???
hypervalent_iodine replied to MigL's topic in Suggestions, Comments and Support
It exists, but has been hidden. -
! Moderator Note This is a science forum. The default position is that of mainstream, accepted science. In this case, climate change is a very real threat to our world, and the fossil fuel industry is a big contributor. In any case, dice your thread amounts to little more than ill-informed rantings, it is being moved to the Trash. If you have your own scientific ideas to present that are outside of mainstream science, post them (along with your proof / evidence) in Speculations. If you would like to discuss aspects of mainstreams science, please do so.
-
! Moderator Note rangerx and Raider, We have been here before. Rangerx, I personally am tired of having to intervene in political conversations that you are a part of. Please learn the value of civil discussion and reconsider the way by which you talk to members whose beliefs who may not agree with. You constantly generate hostility in these threads, throw insults, and drag things off topic with hyperbole. It will not be tolerated. Raider, as you well know, responding in kind does you no favours.
-
Students doing the bulk of the bench work (which includes contributing ideas as well as performing experiments) is par for the course, though. Once you get to the point where you are in charge of your own group, you tend to be more of an administrator than an active bench scientist. The higher you go the truer it becomes.
-

Trigger happy mods?
hypervalent_iodine replied to QuantumT's topic in Suggestions, Comments and Support
What thread are you talking about?