-
Posts
4586 -
Joined
-
Last visited
-
Days Won
12
Content Type
Profiles
Forums
Events
Everything posted by hypervalent_iodine
-
Okay, but the stuff about electronic configuration doesn’t really do that. If you don’t have to go through the process of determining oxidation state, then it seems to be enough (based on the screen shot you sent of the ref page) to simply say that iron has several oxidation states, but exists as Fe3+ in Fe2O3.
-
How you wrote it is fine, but as I said, I would remove some of the stuff about electronic configuration as I don't think it's needed. I have crossed out what you don't need below. You might just add a sentence saying that the iron in Fe2O3 is Fe3+. Also, this is a side note, but I believe iron can also have a 6+ oxidation state.
-
But you weren't told if it was ferrous or ferric, just given the molecular formula. That is why I am not sure how you are expected to know that it is iron(III) without actually calculating it. Anyway, it doesn't seem to matter. Based on their solution, what you have written is fine, but I would get rid of all the electronic configuration stuff.
-
Okay. It's not totally clear to me if you also need to show how you worked out that the oxidation state of the iron was 3+ or if you can just take that as given. Most naming questions that I see will tell you the oxidation states if it's ambiguous. Since it's not explicitly stated here, maybe you do need to give working for that part as well. Regardless, this part in your original answer: Is not really needed / relevant. If you do need to show how you figured out that it is Fe3+ and not Fe2+, how would you do that? You've said that it is Fe3+, which is right, but I'm not clear on exactly how you worked that out or if you just guessed / looked it up. Could you elaborate on this part? I can go through it if you don't know how. I should also ask how you have been shown to determine / calculate oxidation states of atoms in a compound.
-
Looking back on your question and the flow chart, I think I might be getting you to explain a more complex concept that you don't need to worry about in this instance. Your explanation in your OP is fine based purely on that. Have you been learning how to assign oxidation states?
-
More or less, yes. Introductory chemistry is taught in a lot of different ways, some good, some bad. In Australia, teaching content is not standardised and teachers can essentially pick and choose what they want to teach and how they want to teach it (within reason). This can be good, but it also makes the lives of course coordinators at universities difficult when you have to deal with classes with such a varying base knowledge. I, for example, had never been taught how to calculate pH when I got to university. Honestly, I wouldn't have a problem with using Lewis dot notation for bonds if it were only used within that one context for teaching students about constructing Lewis Structures and if I didn't know how ingrained that notation can become. That being said, it can get very messy when you start doing it for atoms with more than four bonds (PCl5 for example). I've marked and taught first year pretty extensively for I guess the last decade now, and even after an entire 1-2 semesters of being told not to draw structures like that, we still see a significant number of students drawing out reaction schemes with dots for bonds. The one I actually dislike the most is the use of a line to represent a lone pair - it looks like a negative charge! Yet without fail, every semester, despite never having been taught in first year to show lone pairs like that, there is always a handful of students who do it.
-
Not sure what you’re talking about here. My method isn’t a bastardised version; it’s how chemists draw structures when including lone pairs. It’s how Rachel (correctly) drew her structure in her first post. What I was saying was that drawing covalent bonds as electron dots is a bad habit (eg C::O instead of C=O). I didn’t say anything about lone pairs. Without the lone pairs it wouldn’t be a Lewis structure. Sorry if that wasn’t clear in my posts.
-
There are a lot of things we believed or did in chemistry in 1916 that we no longer do and no longer teach, for good reason. “It’s the way Lewis himself did it,” isn’t a good enough reason to teach students notation that can cause later confusion more than 100 years after the original publication came out.
-
Why is it not 2+?
-
Still not really what I’m getting at. That doesn’t explain why, based on the molecular formula, you have Fe3+ and not 2+. How did you determine that?
-
The only addition I would make is to include a sentence that states that the Lewis structure does obey the octet rule, since both the carbon and oxygen have 8 electrons around them.
-
No, I mean the math on how you got to +3. Why couldn’t it be +2?
-
“The rules of drawing Lewis structures,” includes having the correct number of electrons. Given that, and given everything else we have discussed, how do you think you should write it?
-
That’s right. If you only have 12 valence electrons available to make up the structure, as you do, you can’t then wind up with 14 electrons. So the Lewis structure must be wrong (too many electrons!). To get to 12 electrons, we have to put in a double bond. Once you do that and you count the number of electrons again, you should have the right number. Have a look at the structure in your very first post with the correct Lewis structure and count the electrons.
-
There doesn’t seem to be any reason to include the electronic configuration in your answer or to go through how iron can exist as iron(II) or iron(III). You simply need to determine the oxidation state from the molecular formula you are given. The reason the have that second step in the flow chart is because some elements like iron can have multiple oxidation states, which means that unlike elements like oxygen or hydrogen, you can’t immediately tell what it is without doing a little math first. It’s not asking you to explain how an element can get those states based on its configuration or even where the electrons go / come from, which is what you have done. What I would include is the math for how you determined that the iron in Fe2O3 is iron(III). You’ve written that it has a 3+ oxidation but not how you got to that point.
-
No. This was wrong in your first answer and it’s still wrong here. Both the carbon and the oxygen in the Lewis structure in the question have 8 electrons around them. Both satisfy the octet rule. I’m not sure how else to explain this, so perhaps I will work on something later and you can review the posts in this thread in the meantime. Yes, but how do you know that? Saying that carbon has to have four bonds isn’t good enough reasoning, because that statement isn’t always true. Same for oxygen. Please go through the post I made in response to MigL. It works through this aspect of the problem. Great! So what does this tell you about the Lewis structure? My comment was made for two reasons. Based on the OP, Rachel is perfectly comfortable using lines to represent bonds and can tell that each bond has two electrons in it. The second reason is that it is not standard notation and when you get to later topics or even when you get further into drawing Lewis structures, using dot notation to represent bonds is confusing. Many higher institutions will mark you wrong or deduct marks for using this notation - it is a bad habit to fall in to. It is truly the bane of our first year teaching staff’s existence. They take this notation from this topic and start drawing bonds as dots when covering, for example, organic chemistry. We have systematic methods of drawing structures for a reason! The other rather irritating part is that it is taught differently and inconsistently in different schools and so there is a lot to have to stamp out.
-
Sorry, I seem to have caused confusion or you have misunderstood me. When I said you only count the valence electrons once, I meant when you look at the Lewis structure and you are tallying up electrons you only count each electron once. You still have to compare that number to the total number of valence electrons from the molecular formula. When you are determining whether or not the octet rule has be obeyed, you have to look at each atom separately and look at how many electrons are around it. The oxygen has 6 in lone pairs and 2 from the bond to the C, which makes 8. The carbon has 2 each from the bonds to the hydrogens, 2 from the bond to the oxygen, and 2 from the lone pair, which again makes 8. Each hydrogen has 2 electrons around it from the bond to the carbon. They all check out. Then you have to count all of the electrons in the bonds and lone pairs and see if they match with the total number of valence electrons as per the molecular formula. You’re half way with this. You now need to count up all of the electrons in bonds and lone pairs in the Lewis structure (these are the valence electrons in the Lewis structure) and check that it matches with the number of valence electrons you have calculated from the molecular formula. Is it the same number? These are two separate actions and you should do both to check that the Lewis structure is correct.
-
I wouldn’t call 5 kg a small scale by any stretch. I can have a look tomorrow, but you could try Oakwood if you’re located in the US. There are other companies that specialise in small heterocycles that might be worth looking at, but 5 kg is quite a bit and I wouldn’t expect it to be cheap. Maybe not sigma prices, but not too much under that. You can sometimes find random Chinese companies that will do it for much cheaper, but you pay for that at your own risk. If you have access to SciFinder, the supplier search function can be useful.
-
I’m still not totally getting your question. There are two things you have to figure out once you have the basic structure down 1) is the octet rule satisfied; and 2) do you have the correct number of electrons? Counting the electrons around each atom in the Lewis structure will help you determine whether or not the octet rule is satisfied. Tallying up the total number of valence electrons based on molecular formula and comparing that to the total number of valence electrons in the entire Lewis structure will tell you if you have too many or not enough electrons. How many electrons are in the Lewis structure of your compound? How many should there be? Is the octet rule satisfied?
-
I don’t totally understand the question. You have to check both things - the total number of electrons and whether or not the octet is satisfied. They’re related but separate things in the sense that one being correct doesn’t mean the other will be.
-
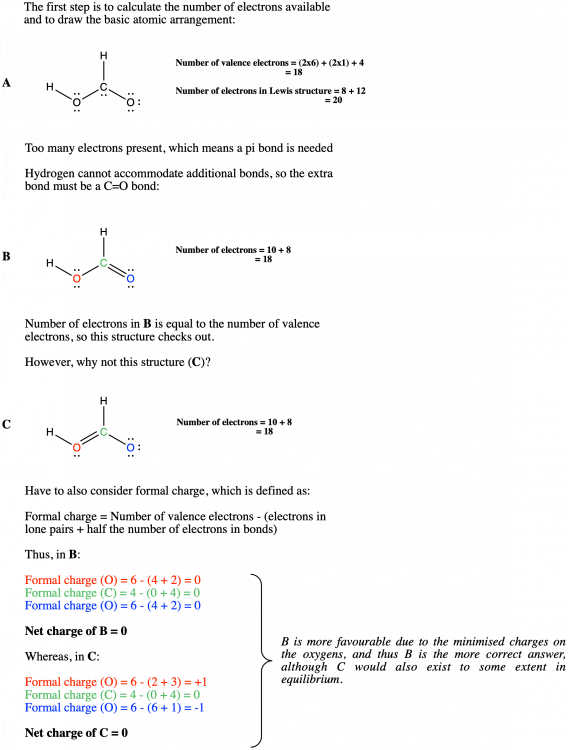
You need to do two calculations here, one where you calculate the total number of valence electrons available, and other where you count all of the valence electrons in the Lewis structure (i.e. all of the ones in bonds and lone pairs). If the Lewis structure is correct, the numbers must be the same. If we look at my previous example in my post of formic acid, HCO2H. Each hydrogen has 1 valence electron, the carbon has 4 and the oxygens 6. Thus, there is a (1x2)+(6x2)+(4x1) valence electrons, which comes to a total of 18 valence electrons available. If we then look at the number of electrons present in structure A, we have 6 lone pairs and 4 bonds with 2 electrons in each, which is equal to a total of 20 valence electrons in the Lewis structure. The numbers don't match, so the Lewis structure (A) is wrong. See if you can apply that to your molecule.
-

Can someone please detail how epigenetic changes happen
hypervalent_iodine replied to Angelo's topic in Biology
I believe you are asking about epigenetic inheritance. There are papers on this. It is a rather broad topic. If you have access, this paper is a good overview. You may also like to look at the wiki article. -
Yes, they do. However, the Lewis structure is still incorrect (for other reasons). Have you counted the total number of valence electrons in the Lewis structure? How does that compare to the total number of valence electrons available?
-
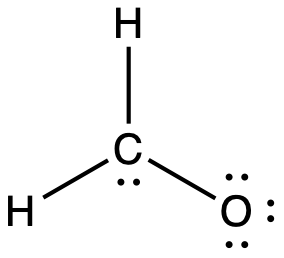
No. I think the problem you are having is that when you count the two electrons between the carbon and oxygen in the question, you are only counting them once for one of the atoms and then treating it as though that atom has sole ownership over them, which is not the case. The question you have to ask yourself when considering the octet rule is how many electrons is each atom surrounded by individually. You are not asked to consider how many electrons the atom possesses in a formal sense (i.e. both electrons from a lone pair and one from each bond), just how many electrons are around it total. Look at each of them separately, count two electrons for each bond that atom has and two electrons for each lone pair. For carbon, yes, that makes 8 (2 from the lone pair and 6 from each bond). Similarly, for both hydrogens you should count 2 from the bond to the carbon. However, that also makes 8 for oxygen (6 from lone pairs and 2 from the bond to the carbon). Note that this double counting is only when you are looking to see if the octet rule has been satisfied. If you are then counting the total number of valence electrons, you obviously only count the electrons once. Does it help you to visualise it if the structure from your question is redrawn like this?:
-
Spot on. This is more or less what Rachel drew in the picture in the OP, except using the (much more correct) bond notation of lines instead of dots. Many high schools will teach students to draw bonds using dots and x's when teaching Lewis structures, but it is an awful habit and can lead to confusion later on. We will usually deduct marks from students for using this notation when they get to first year (they are told not to). In any case, I don't believe knowing how to draw the correct configuration is the issue here. You have hinted at the real problem with Rachel's description in this sentence: The way students are generally shown to think about this is by adding up the valence electrons, and matching that up with the Lewis structure. So I don't completely give the answer away, we can consider formic acid (HCO2H) as an example: (Image mine, drawn in ChemDraw) It becomes slightly more complicated when you get to hypervalent compounds (such as (bis(trifluoroacetoxy)iodo)benzene, which is where my username comes from), but only in the sense that you have to consider the possibility of 10 or 12 valence electrons. These are covalent bonds, no one is suggesting that they are ionic. Look at the structure again and count the number of electrons around the carbon and then the oxygen. How many do you see?