-
Posts
4586 -
Joined
-
Last visited
-
Days Won
12
Content Type
Profiles
Forums
Events
Everything posted by hypervalent_iodine
-

Thread Hijacking and Staying On Topic
hypervalent_iodine replied to swansont's topic in Suggestions, Comments and Support
! Moderator Note RB, While you may have inspired this thread, this is not a conversation that was directed specifically at you. It was a thread intended for general advice on an issue that has been seen rather a lot lately. If you want specific advice on specific moderation of a specific thread, PM someone or open a new thread. Whatever you do, you are to stop derailing this discussion. Do not respond to this mod not within the thread. My irony meter just can't handle this level of abuse. -
High concentrations of DNA are known to inhibit PCR. There are a few possible reasons that I can think of for why. The first is that your primers may be binding to other sites on the template strand. High concentrations of DNA with the same primer concentration would mean that more of your primers are binding to sites other than your target region, and you either aren't getting amplification, or you're getting amplification of the wrong bits. If concentration is super high, the diffusion of Taq through the tube could also be blocked, making the whole thing inefficient. I think that DNA is also an inhibitor of polymerase after a certain point. Could be wrong on that, but I recall hearing it somewhere once upon a time.
-
You've got the first part towards the answer, but you have to consider the rest of the question. It has stated that the mass percentage of P2O2 in a fertiliser is 30% and the question has asked you, with this information, what would the mass % of only phosphorus be in the fertiliser. You have calculated the mass % of P in P2O2, which is good, but you then need to carry that number on to work out the % in the fertiliser as a whole.
-
You don't divide in this case, you multiply. It wouldn't make sense to divide, as your number would then be less than 1.
-
It's not a partial pressure question. Consider the number of moles in the given amount of hydrogen and how many miles of deuterium you would need to achieve the same pressure, as per the ideal gas law.
-

self-disproportionation of N-acetyl-dl-phenylalanine
hypervalent_iodine replied to Maniek's topic in Organic Chemistry
Have you tried SciFinder, Google Scholar or Web of Science? -

How to get artemisinin-transferrin conjugate
hypervalent_iodine replied to Wlas's topic in Biochemistry and Molecular Biology
! Moderator Note This is really something you should be doing in full consultation with a medical professional. It is not appropriate for us to be advising you on how and where to obtain something you plan on using as a home brew therapeutic. Thread closed. -
IR is your best bet. It's quicker than NMR, and solvents like CDCl3 can cause problems if you have very unstable ones. The isocyanate peaks are pretty obvious as well. Colourful substance can often mean it decomposed. For reference, my ones would go bright red when they broke down. Unless you mean a chemical test? I suppose a ninhydrin test would tell you if you still had amine in there, but that doesn't say much about if you actually made an isocyanate. One way would be to try and do the next step one pot and see if you made that. I'll respond to your last post later on. I've been pretty sick the last few days and am in need of more rest.
-

Unknown Compound HNMR, IR, CNMR and Mass spec HELP??
hypervalent_iodine replied to steelhead's topic in Homework Help
Those problem sheets bring back memories. Pretty sure we used the same ones at the uni I went to. Start with your mass spec and work out the molecular formula. They give you a molecular ion m/z with an isotopic ratio for one of them. This is a big hint. Once you have that, calculate your DBE and go from there. If you can show your working and where you are struggling, we'll be happy to give further direction. -

Amino acid sequence
hypervalent_iodine replied to AlexanderOsaka's topic in Biochemistry and Molecular Biology
Cheaper cost or a fellowship? I'm not sure I follow that request. Are you asking for advice or a job? -
Perhaps. The idea is that the O-tBu is eliminated after formation of the carbamate following a second deprotonation step with base (DMAP in this case, IIRC), giving the isocyanate. I believe the paper I got it from just did a liquid liquid extraction for work up and purification. It was an odd reaction and I was skeptical of it working even though I thought it sounded like a nice alternative. Like I said, I couldn't get it to work. My amine was particularly low on the nucleophilicity scale however, which may have contributed. I'll see if I can find the paper I got it from tomorrow when I'm back in the office. Is your final product after the isocyanate a carbamate? There are other ways to make carbamates that avoid things like triphosgene. Is there any reason you're going this route and not another?
-
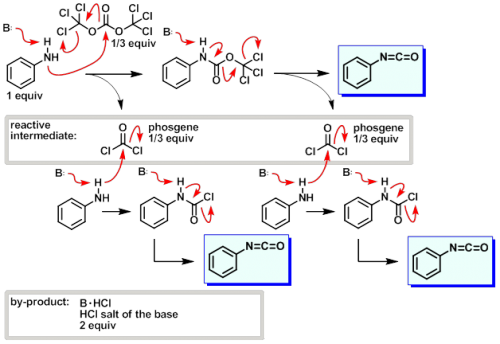
Presumably, the base is there to deprotonate the amine and mop up HCl. Yes, the reaction would be exothermic. Having it cooled helps to control the excess heat and prevent side reactions. You also want to add the amine drop wise as a solution in THF. It should be okay to remove the ice bath after complete addition of the amine. Definitely do it under inert atmosphere. Make sure everything is super dry and that your reagents are dry and of good quality. You may have to do some distillations for this part. My lab has a THF still set up in one of the fume hoods, which makes life easier. Have you looked through SciFinder? I recall finding some protocols using oxalyl chloride there when I was attempting to make isocyanatses. I believe I found it through the reaction search tab using a generic pyrimidinyl amine as my reagent. I also found another method using Boc anhydride, which I thought was pretty neat but could not get to work with my jerk of an amine. What kind of amines are you working with? What's the step after the isocyanate? Edit: here is a decent mechanism From http://drugsynthesisint.blogspot.com.au/2014/02/a-review-and-methods-to-handle-phosgene.html
-

two antibiotic solutions to mix
hypervalent_iodine replied to Silvia_84's topic in Microbiology and Immunology
Unless you've made a typo, it isn't possible with those concentrations. Anything you add to the 10 mg/mL solution will dilute it; you're not able to have both antibiotics at 10 mg/mL with these stocks unless you add the other one as a solid or make up a different concentration of the 10 mg/mL one. I would recommend the latter. To answer your math problem with different stocks, let's say were making up a solution with two stocks of 50 mg/mL and need to get to an final concentration of 10mg/mL. Each would need to be diluted 1:5, so I would simply add 20 or 200uL of each and make up to 100 uL or 1000 uL respectively, depending on how much you have / need. -
I believe that you can use oxalyl chloride, but phosgene is your best bet. Why don't you want to use DiPEA? If you don't have any, I would go to TEA or straight pyridine before DMAP. What are you making the isocyanate for? They are not the easiest compounds in the world to work with if you have to isolate them as they are notoriously unstable. The last time I made some, they had decomposed in the time it took me to carry a small sample down to the IR.
-
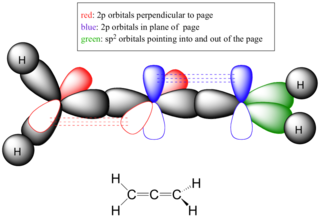
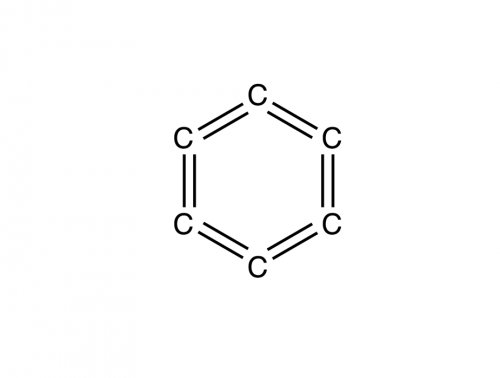
I assume you mean something like this? H-C=C=C-H is a known compound, but you couldn't form a six-membered ring of only C=C=C... You have to consider the presence of pi bonds and what that does to the geometry of the carbons. For example, allene: (Image from http://chemwiki.ucdavis.edu/Core/Organic_Chemistry/Organic_Chemistry_With_a_Biological_Emphasis/Solution_Manual/Chapter_01_Solutions ) As you can see, the central carbon has two pi bonds, one extending to one carbon along the z axis and the other to the second bonded carbon along the y axis. The overall affect of this is that the geometry becomes locked in place and the C-C-C angle has to be 180o. You could never form a ring from cumulated polyenes like this simply because you'd be placing too much angular strain on the bonds and the geometry doesn't allow it.
-
1. You don't need to know it as you're not working in moles. 2. Yes. 3. Yes.
-
Depends on the exact reaction. In addition to kinetic considerations, temperature also affects equilibrium. You can also get different things out by altering the temperature and playing with thermodynamic vs. kinetic control. Increasing the rate can help to improve yield when considering a constant reaction time, also.
-
Pernicious anemia is something that runs in my family. That particular type of anemia is a little different from most - it's an autoimmune disease that destroys the cells responsible for taking up B12. It wouldn't matter if you were were a vegan or a straight meat eater, if you're a sufferer, you're generally not going to be getting enough B12 regardless. Many have to get regular injections. The consequences for not doing so are not enjoyable, even ignoring the eventual death thing. I should also note that plain old B12 anemia is not the same. Pernicious anemia is a very specific condition. The former you can fix by changing your diet or by taking supplements. OP: While I agree that we could do better in the way we treat livestock, I find your argument about the ethics of killing of animals for food to be unconvincing. Why do you define the killing of an animal as large as a cow as unethical, but not say, a cockroach? A mouse? How about a tree? You eventually have to draw a line somewhere, and I'm not sure why your line is any better than mine, speaking as someone who just finished eating a bacon sandwich.
-

Need help understanding acids and bases
hypervalent_iodine replied to Helix-Oxford's topic in Homework Help
The dissociation of an acid in water can be described as follows: H-A --> H+ + A- The reaction exists as an equilibrium for weak acids. The more acidic something is, the more the equilibrium favors the products, or in other words, the more H+ there is in solution. For strong acids, the dissociation goes to completion. This is a little inaccurate. It is the electrons that 'move' to break old bonds and create new ones. -
Feel free to extend my questions to general responses to such threads. I have looked at the following threads in Speculations. http://www.scienceforums.net/topic/87675-hi-im-new-here-looking-for-more-info-on-geocentrism http://www.scienceforums.net/topic/93501-a-probabilistic-proof-of-the-existence-of-etraterrestrial-life/ http://www.scienceforums.net/topic/93637-minimum-emf-wavelength/ http://www.scienceforums.net/topic/93388-black-spring-theory/ http://www.scienceforums.net/topic/93471-local-isotropic-length-transformation-hypothesis/ http://www.scienceforums.net/topic/89968-seeking-help-of-mathematicians-lie-algebra-invariants-group-theory-to-complete-a-derivation/ http://www.scienceforums.net/topic/93076-telekinesis-telepathy-and-their-impact-on-science/ In all of these examples, I think our members made a commendable effort to correct any flaws presented by the OP and ask questions about the science they presented, and I do not think that they were rude about it. In general, the posts by our pre-existing members has been well received by the newcomers and a couple of the threads were, by mere coincidence, excellent examples of what we hope to achieve by even having the Speculations section. Furthermore, I do not think any of them contained premature or unfair / harsh moderation. Is there any disagreement with those statements? Have I missed something, or are there other threads I should be looking at? http://www.scienceforums.net/topic/93673-an-explanatory-attempt-of-our-universe/ This is probably an exception, but the member is not new and the post was not science. The member was given a chance to elaborate, but did not do so. At some point, we do have to draw a line with what we allow and what we do not.
-
You don't seem to have really read what I wrote. Since I have been a mod here, I know of a small handful of threads in the mod forum where we have examined the way that we interact with newer members. We are not shy of recognising potential faults in how we deal with members and trying to remedy those faults, and I am all for standing up and saying that we need to improve where appropriate. However, for us to move forward with this particular assessment, we need to be talking on a common ground. You both say there is a problem, yet no one else seems to agree. There is either a difference in what we consider harsh, or we have missed the examples that have led to this appraisal (or, as you say, we are deluding ourselves). In any case, it would be edifying for all involved if we had some specific examples to talk about. I am sorry that this has you frustrated. I can see where you are coming from in your annoyances with this discussion, even if we don't necessarily agree on the main points. Some further questions regarding how you perceive moderator action with newer members, to help source out where you and TAI think we should make improvements: 1.) Do you see a problem with the type of language used, the length (or lack thereof) of mod posts, or both? 2.) Do you believe that action is taken too early or too late in Speculations threads by new members? (Ophiolite, I know you already answered this) 3.) Given your answer to 2.), what would your approach be to members clearly unfamiliar with how we expect them to be posting there? 4.) What are your opinions of the six pinned threads in the Speculations forum in relation to the OP? I am going to take some time to review the Speculations forum and some of the old mod threads I mentioned.