-
Posts
4586 -
Joined
-
Last visited
-
Days Won
12
Content Type
Profiles
Forums
Events
Everything posted by hypervalent_iodine
-

Need a list of any unique substance you can think of....
hypervalent_iodine replied to MWresearch's topic in The Lounge
How does one calculate the coefficient of friction between fire and sunlight? Mountain and treasure? Liquid and....anything on that list? Can you see why I am struggling to see its usefulness? Also, no. We are not making strict guidelines or rules for your own list, that's your job. And we sure aren't making this a sticky. -

Need a list of any unique substance you can think of....
hypervalent_iodine replied to MWresearch's topic in The Lounge
I'm not ignoring anything, I'm telling you that I cannot think of a way in which your list could possibly be of use to chemists. Your one example of how it might be useful tells me that you have no idea what chemists actually do. -

Need a list of any unique substance you can think of....
hypervalent_iodine replied to MWresearch's topic in The Lounge
Chemists have very specific means of doing that and you can rest assured that a list that includes fire as a substance will be of absolutely no use to them. -
Love Audible. I use it to listen to books in the lab if I'm doing something especially monotonous. Makes the bus more pleasant too, since I can't read on buses without getting sick.
-

Need a list of any unique substance you can think of....
hypervalent_iodine replied to MWresearch's topic in The Lounge
! Moderator Note Your thread is about an arbitrary list of things that your "average person" knows about. You're hardly interested in "scientifically established substances" (whatever that's supposed to mean) at all, or John Cuthber's suggestions wouldn't have been so disagreeable to you. This doesn't have anything to do with actual science, unless there's something you're not mentioning in this thread, so it hardly belongs in that part of the forum. Furthermore, discussion about whether or not you can edit the OP or where this thread has been moved are not the topic of discussion. If you have a problem with anything of that nature, you're more than aware of where to take your complaints. Do not respond to this modnote in the thread. -

Do Deaf People still have the reflex to breathe?
hypervalent_iodine replied to LisaLiel's topic in Biology
! Moderator Note LisaLiel, this topic was dealt with. Do not reopen it. -
! Moderator Note Mike, this has to stop. We are constantly having to reprimand you for your inability to maintain a coherent discussion and frankly, it is growing tiring to have to wade through these threads to decide whether or not they need to be closed. If someone responds to you with a valid point that refutes what you are saying, you need to start making a much more concerted effort to actually reply to them in kind. It is not an invite for you to start rambling on about irrelevant stuff about the universe or whatever it is that has taken your fancy. That is not how a discussion works and it's about time you learned that. Since you have once again started to invoke elements of a closed discussion, I am closing this thread. In the future, you need to stick to the thread of conversation. If someone raises a valid argument against what you are saying, respond only to those points. Do not go off on tangents. Do not soap box. Do not bring up closed topics. We are going to be much more stringent on these issues in future and if you cannot comply, do not be surprised if you find yourself suspended.
-
! Moderator Note Another reminder to all members that this thread does not ask questions about whether or not there is a God or an afterlife. Please stick to the topic.
-
! Moderator Note metaman, please stop hijacking threads with such nonsense. seriously disabled, insulting other members is not tolerated here. Do not do it again. Do not respond to this note within the thread.
-

Help with insect larva identification
hypervalent_iodine replied to pavelcherepan's topic in Biology
In my younger years, I used to go around looking for click beetles for the purpose of making them click in my hand and jump around. They can reach some pretty amazing heights for something so tiny. -

Stoichiometry, the bane of my existence.
hypervalent_iodine replied to Dovahkiin's topic in Homework Help
Look, to each his or her own. If the OP finds it better to work that way, more power to both of you. I'm not even trying to say that your method is bad, I'm simply saying that it is unlikely to be similar to how the OP has been going through questions in class and is thus could lead to confusion for them. No high school / first year / any chemistry class (that I have encountered) will teach you to change the coefficients in a reaction from moles to grams. That simply doesn't make sense in that context. -

Stoichiometry, the bane of my existence.
hypervalent_iodine replied to Dovahkiin's topic in Homework Help
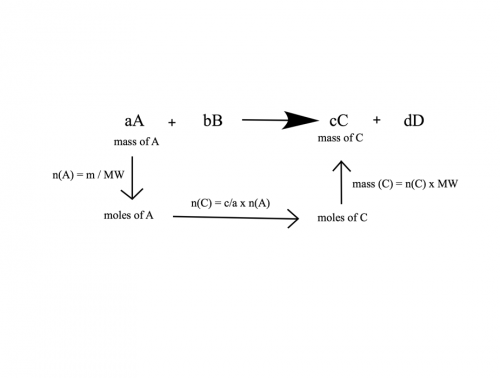
Again, to clarify some things. I know studiot has said that gram-molecule is the same as saying mole, but the term we use in chemistry is always moles. It is best to stick to that. Since this is fresh material for you, I would again recommend sticking to a step-wise approach. If you have a process that you use for every question of this type that is unambiguous and that you understand, you will not have a problem answering these questions. You have been given a number of the-same-but-different approaches here, however if you are more of a visual person, I came across this diagram (I've had to draw it myself, but you get the idea) in a student's text book a few months ago that I quite liked. It more or less summarises what I said in my last post. The above is just an example. If the units of A were something else, substitute the first arrow with the appropriate equation to convert to moles. And of course, the method could be applied to any combination of A, B, C and D; it depends on what your question is. Personally, and I do not mean this to disparage studiot, I think you are considerably better of not using his method. It will give you the correct answer, but changing the coefficients in your reactions from moles (as given in the reaction - 1 mole of Al(OH)3 reacts to make 3 moles of water) to grams is a round-about and confusing way to answer your question. It will also be unhelpfully complicated for you if the units you are dealing with are, say volume instead of grams. Stick to moles and convert to your desired units at the end. It's much simpler and more than likely, much more compatible with how your teacher will be going through it. Finally, to reiterate what I said before, if your question is only asking you to account for the amount of one of your products or reactants given the only one other component of your reaction, it is not necessary to calculate everything. It is certainly good practice while you are learning and as studiot rightly points out, a good way to check your results. If you are confident in your process, then doing this may only serve to increase the amount of time you are taking to answer questions in an exam and clutter your exam sheet in the case of short answer questions.* *I have mentioned this to pavel already, but I also wanted to mention quickly that I am only including this clarification because my experience is that showing students how to answer a questions with a load calculations that aren't strictly in answer to it can sometimes be confusing for them. It is probably an unnecessary comment to have to make, I am not sure, though I think it is best to be safe and as clear as possible as to how our explanations fit in with the OP. -

Stoichiometry, the bane of my existence.
hypervalent_iodine replied to Dovahkiin's topic in Homework Help
What pavelcheperpan has said is correct for your question, though I would clarify that you multiply only by the coefficient of the product you want, rather than all of them. The general process is to convert the mass or volume, etc., of what you have into moles, multiply that number by the ratio (the coefficient of the thing you want / the thing you have; in your case 3/1) to get the number of moles of your product or whatever it is the question is asking for and then convert that number into whatever units are required. -

Will a person suffocate if their inner ears are destroyed?
hypervalent_iodine replied to LisaLiel's topic in Biology
! Moderator Note I think we're done here. LisaLiel, as your questions concerns the health of your brother, you should be seeking proper advice from a professional. The Internet is hardly the place for it. -

2016 US Presidential Race - Landslide or Laughing Stock?
hypervalent_iodine replied to iNow's topic in Politics
Rand is less boisterous than some of his counterparts, but I'm skeptical of the opinion that he is less extreme in some areas. Prior to a year or so ago (when it was becoming clear that he would probably run), his views on certain social issues were just as extreme as any of your best tea party members. The difference is that he's tried to pull back from those positions in the past year or so, which I rather suspect is to garner more of the middle-of-the-road votes and make him a more attractive candidate for a general election. Personally, I believe him to be extremely disingenuous and I find it hard to believe that his flip-flopping on social issues is because he's genuinely changed his thinking on matters. As I said a while ago in this thread, however, I doubt he'll get through the primaries. I still, don't know who I'd prefer on the republican ticket. They're all just as terrible as they were last time I commented here. Cruz and Bush seem to be the figureheads for each end of the Republican spectrum, so I wouldn't be overly surprised if it ended up being between them. That being said, I liked Scott Walker for it for a while and although he's been a bit quiet in the last few weeks, I'd still put him at or near the top in terms of likelihood to win the nomination. -

What's new in physics (split from is Nobel suffocating science)
hypervalent_iodine replied to Chriss's topic in Physics
You realise you could just look these things up yourself, right? If you're interested in knowing what's going on in science, your best way forward is to do some reading. -

Will a person suffocate if their inner ears are destroyed?
hypervalent_iodine replied to LisaLiel's topic in Biology
! Moderator Note LisaLiel, I have merged your two threads on this. Please stick to one thread per topic. -
I think we could all do with a reprise from the hail of foul language and abuse from Questionist. He has been suspended for a week to cool off and maybe read the modnotes he was so keen on ignoring. ---------------- randomc was suspended a few days ago for a week. This wasn't updated at the time because we were considering a ban.
-
! Moderator Note Questionist, A number of your posts in this thread are simply unacceptable. You don't get to tell people to stop posting in a thread and you certainly don't get to do it in such an uncivil manner. You also need to stop making plainly ridiculous assertions if you don't plan on actually backing them up with evidence. Mocking established science, etc. because you happen to think it ridiculous falls under the banner of logical fallacy, which our rules strongly discourage. If you can't do this, then this will be closed. Do not respond to this note in-thread.
-
You should think about it retrosynthetically. Have a look at the molecule and see if you can think of an obvious disconnection. As a hint: bonds to heteroatoms are a good place to start. It would also help if you could elaborate what level your student is at and what sort of reactions they've been learning about, since there will be ways to synthesise this molecule that they may not have been taught.
-

Was this a miracle or a mistake?
hypervalent_iodine replied to Robittybob1's topic in Suggestions, Comments and Support
Apparently, yes.