-
Posts
3887 -
Joined
-
Last visited
-
Days Won
1
Content Type
Profiles
Forums
Events
Everything posted by Enthalpy
-
Chemsketch is free, yes. The edition I use knows only one rudimentary model for chemical bonds. Full of nice small features, like "automatic" draw from a Smiles. ArgusLab is free as well and knows more evolved models for atomic bonds. Using both isn't bad. Though, both allow to draw very well molecules that are completely impossible, say because of strain. This polycylic-pentane frenzy is accepted by both UFF and AM1:
-

How electricity is generated by silicon in solar cell ???
Enthalpy replied to Parshotam's topic in Inorganic Chemistry
Tiny update: the electron excited by the photon comes from the valence band, hence is not attached to one atom. It's broadly delocalized over many atoms. After excitation, it's in the conduction band, which isn't quite the same as free; for instance it mass differs from an elelctron in vacuum. -
The distance at which strong interaction is positive is known - but not for proton and proton I guess. Have a look at Yukawa, and at the tunnel theory of alpha radioactivity. And because fusion involves tunnelling, this distance must be shorter than what the temperature provides; on the other hand, this scarce interaction can and does relies on kinetic energies that are several times bigger than the mean value: again a statistical tail. Pair production prior to emission of a positron? I don't feel it necessary. With gamma rays, pair production is rather efficient when the photon energy suffices, but it is very sensitive to the atomic number: efficient with lead, very poor with hydrogen and helium, whose near electric field is weaker. Electron capture: my suggestion is only that electron capture does happen (in the beryllium-7 example) with an electron density (from the 2s shell) comparable to the electron density of the plasma in a star core, so a temporary di-proton, or an almost di-proton, could use this process just as 7Be does. This could help fusion just as muons are known to do. Would this process be a significant competitor to the complete formation of a short-lived di-proton which then transforms quickly enough into a deuteron? No idea. The answer must be already known.
-

what is the chemical substance which destroyed my banana ?
Enthalpy replied to fresh's topic in Applied Chemistry
Agreed! It's just a heavy trend on forums. -

To make new music instruments for to learn acoustics?
Enthalpy replied to science4ever's topic in Amateur Science
At the mouthpiece end, modelling clay provides easily the taper. As a bell, you may take the neck of a plastic bottle, one with a long neck, and cut slits in the length direction. This allows to adjust the small diameter to your tube, before sealing off with adhesive, glass fiber and epoxy... Only some tones extremely unstable:it can result from - Small leaks, which act differently on varied notes, just as if they were overblow holes - Big obstacles to air movement like steps in the bore. Similar effect. This is used in some saxophones at the neck for a softer sound, in all saxes at the mouthpiece to dampen very high resonances and avoid the reed to whistle - Badly aligned resonance modes. Playing one note excites many modes; the ones almost aligned made the sound wobble and the note difficult to play. This is one difficult part of instrument making; no quick and easy fix, it need a complete design; and if using a tapered mouthpiece on a cylindrical bore, this worry will appear. To determine where to put overblow holes: -Have a small microphone, or a really tiny tube, preferably of metal. Play the desired note (two people if possible) and insert the sensor to find positions (there are many on a brass instrument) where pressure oscillations are minimum, as you don't hear the sound through the pipe. At these places, you can have an overblow hole for the desired note. - But you don't want one hole for every note... Some holes can be common to several notes. As well, some notes can benefit from several open holes. - The baroque trumpet needs many overblow holes because its modes are so close to an other. Woodwinds, which use lower modes, can have few overblow holes. -

what is the chemical substance which destroyed my banana ?
Enthalpy replied to fresh's topic in Applied Chemistry
Someone appropriates the ideas of someone else? It's about bananas here, not about Wikileaks. Maybe the original poster just wants us to invent or suggest a chemical that stops fruits from ripening. This would have obvious uses. While I personally have nothing against commercial applications, I find underhand attempts to conceal the real goal ridiculous and counterproductive (though we may doubt the present thread is such one). Alas, it's becoming increasingly common on forums. -

How electricity is generated by silicon in solar cell ???
Enthalpy replied to Parshotam's topic in Inorganic Chemistry
Silicon (single-crystal, polycrystalline, or amorphous) is only one possible material. Other semiconductors are used, and organic dyes look very promising to replace semiconductors. -

To make new music instruments for to learn acoustics?
Enthalpy replied to science4ever's topic in Amateur Science
Any tube can work as a musical horn. A mouthpiece is not mandatory but helps a lot - provided it matches more or less the tube's length and diameter. Already a tiny smooth divergent at the open end (try a fitting bottle neck) helps a lot: nicer sound, easier emission. But to get properly aligned resonant modes, you need the accurate variation of tube inner diameter over the position. First to produce all harmonics (a cylinder makes only the odd ones) then to put these harmonics where they are to be. By the way, modern instruments don't use the fundamental mode normally ("pedal mode") and this one can be, and is, brutally out of tune. Piston instruments begin with the second mode and have no definite limit, well over the eighth mode. As higher modes are less spaced, a few pistons can join these modes. The baroque trumpet uses even higher modes because they're even closer to an other; to be checked, maybe it begins at the fourth mode. This was necessary without pistons, to make many notes available. The difficulty is to play the desired mode among all these close to an other. To ease it, modern baroque trumpets are still very long for the pitch, hence use high modes which are close to an other, but have small holes that work much like overblow holes in woodwinds, in that they prevent some vibration modes (Wiki is false on that), making it easier to catch properly the others - which are chosen during play by covering only some holes. Even harder then, musicians used the lips to play the missing half-notes: difficult, and the tone quality differed on these notes. Same for natural horns, where musicians closed more the tube with the hand to make the half-notes, but this changed the sound quality. From that epoch dates the (presently mis-) conception that tonalities sound differently from an other. Pistons have eased all this, and allow to play open and "stopped sounds" for all notes. If you find Berlioz' "traité d'instrumentation" (Treatise on instrumentation), he lived at the transition time and explains it. -

Embedding images in sound files and high frequency headaches?
Enthalpy replied to howlingmadpanda's topic in Amateur Science
44kHz is the sampling frequency of many digital sounds, meaning that it can't be reproduced as a sound. Anyway, you wouldn't hear it, nor be affected by it at any reasonably achievable loudness. Images spelling? What does that mean? Of all software I have to display images, I expect none to direct a sound to the loudspeakers. -
Take a microphone, a headset, build an high-gain amplifier. If you hear the hum more loudly in the headset, then the source is not in you. Do you recognize the hum of a transformer? Just go near to one and listen. 50cm from a 1MVA transformer, or 50mm from a 100VA one - plaese don't forget the insulation.
-

what is the chemical substance which destroyed my banana ?
Enthalpy replied to fresh's topic in Applied Chemistry
What I've heard is that mass-produced bananas are picked very early, transported green, ane they "ripen" in the port of the consuming country with ethylene. I dare to doubt that ethylene on cut fruit has the full effect of light on the fruit under the tree... But the alternative is to have the tree in your garden. Once I ate a mango picked ripe minutes before: the difference is astounding. -

Electron injection into a clump of Tungsten, ideas?
Enthalpy replied to Zangetshumody's topic in Chemistry
One of the many theories about cuprates is that some atom layers give or take electrons to the layers that superconduct. As well, silicon could be made superconductor when super-concentrated doping was achieved. (For those interested: http://www.physforum.com/index.php?showtopic=22292, inject holes without doping) Hence my question, if you try to make tungsten superconduct by providing it more electrons. From the available differences of extraction potential, and from an arbitrary permittivity of 5, the maximum distance between an electron donor and tungsten would be one atomic layer (in which case permittivity loses sense). So the only hope would be an alloy where tungsten atoms, or at most, monoatomic layers of tungsten, are surrounded by donor atoms. This is the case in cuprates and oxypnictides, where for some crystal orientation, all atoms of one type are in the same plane, and are surrounded immediately by different atom planes. You'd have to reproduce a similar crystal arrangement with tungsten. Whether we can call metallic electrons 5d... Electrons do what they want. Letting orbitals interfere is only an approximation, and restricting the sum to 5d is an arbitrary choice. -
You were lastly speaking about water molecules, in which context I replied. A metal is different. "Conduct heat at zero kelvin" is a formal paradox. A question of logic, not physics. At arbitrary low temperature, electrons conduct electricity, and very well. Again your old misconception. Why stick to it? And again, heat conductivity is low because these very mobile eelctrons store little heat, proportional to kT. exp(ikx) exists at any temperature, even cold, and many such states are occupied. The unique state with k=0 is negligibly scarce. The states in a metal are usually not called "orbitals", and besides naming, their states are delocalized to the full metal part. Your old misconception, again. Well, I stop for this time. A metal is not a small molecule, and you have to understand that electronic states are not bound to one atom, but spread over all atoms. These states are the lowest accessible ones to electrons and are occupied at any temperature.
-
At moderate temperature, electrons are not excited. [by the way, hydrogen has no 1s and 2s in water - only molecular orbitals] Heat is stored in translation, rotation, in PV (for the Cp heat), little in vibrations at room temperature. In the liquid, breaking more hydrogen bonds at a higher temperature makes a significant heat capacity, which you can estimate by comparing the liquid's capacity with the vapour's Cv (or Cp - P*V). Heat is very unlikely to excite electrons, even in molecules bigger than water. Dyes for instance are specially made: long and with electrons delocalized over dozens of atoms; and then the excitation energy is in the visible spectrum, which corresponds to thousands of kelvins (Sun's chromosphere: 6000K).
-

Electron injection into a clump of Tungsten, ideas?
Enthalpy replied to Zangetshumody's topic in Chemistry
Would you tell us what the use is for your 5d-enriched tungsten? Is this a current hot research topic? Superconductor, photoelectric, catalyst, laser...? -
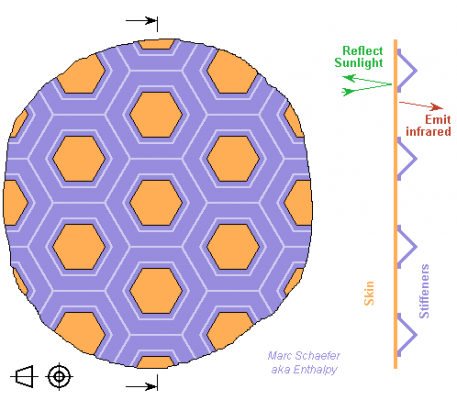
Usual honeycomb sandwiches with 0.3mm aluminium skins are heavier than desired for the concentrator. Space technology has probably lighter ones. Here I suggest a different process. Nickel parts are produced down to 8µm thin for elastic couplings; here 50µm shall provide enough local stiffness, with features about 20mm wide for ~80mm periodicity, as an experience-backed feeling. A few additional parts shall provide the global stiffness; consider brazing. The skin is deposited on a mould that gives the concentrating shape and preferably the optical smoothness. The reflecting layer may be deposited before. Then a thick layer is deposited temporarily on the skin and patterned to the hills and valleys; rocket cooling jackets use wax for that. The nickel stiffeners are deposited on the skin and the hills. The temporary layer is removed. A rear skin could be added on the stiffeners using a new temporary thick layer to produce a complete sandwich. This can be lighter if the skins get thinner. Once the several layers achieve enough stiffness, the reflector can be removed from the mould. Other parts can be produced this way, for instance antennas, or Sunlight concentrators to produce electricity. Other possibilities exist, for instance a sandwich of metal skins and thin balsa wood. The heat conductance suffices, but I like the all-nickel contruction for uniform thermal expansion. The Astromesh antenna is big, unfoldable, and its weight would nearly fit, but it's not a precise and efficient optical reflector up to now. Marc Schaefer, aka Enthalpy
-
¡Hola compañero! Welcome here! http://en.wikipedia.org/wiki/Magnetic_refrigeration#Working_materials I quote: "Recent research on materials that exhibit a giant entropy change showed that Gd5(SixGe1−x)4, La(FexSi1−x)13Hx and MnFeP1−xAsx alloys, for example, are some of the most promising substitutes for gadolinium and its alloys — GdDy, GdTb, etc. These materials are called giant magnetocaloric effect (GMCE) materials." and: "The originally suggested refrigerant was a paramagnetic salt, such as cerium magnesium nitrate." this one seems to be for low temperature. Same or worse for nuclear demagnetization. Introduction: http://materialsscience.pwr.wroc.pl/bi/vol26no4/articles/ms_2007_01Szymczak.pdf Last time I vaguely dreamed of magnetic refrigeration, I wanted to have tiny ferromagnetic domains; spaced enough in a matrix so the global material would be just short of ferromagnetic at the working temperature. Anyway, the absorbed heat is small. No idea if it's a sensible way. Maybe you could experiment with any material just above its Curie temperature? For instance Mn-Zn ferrites are commonly used in electronics. It's just that cooling from hot temperatures needs nothing special...
-
Elastic rubber is described in every article about elements. What sort of answers, questions, comments do you expect?
-
What lets you imagine that you obtain CO2 but no CO? Why H2O but no H, H2, OH, O2? Have you considered the temperature of this reaction?
-

Platinum and Iridium Electrodes in Fuel Cells
Enthalpy replied to njaohnt's topic in Inorganic Chemistry
Graphite is an assembly of molecules, not an atom. -

what is the basic reason that Al does reacts with nitric acid ?
Enthalpy replied to Parshotam's topic in Inorganic Chemistry
Resistance to oxidation is very complicated, completely experimental, and explanations are better sought after the observations are made... Few aluminium alloys resist corrosion. Al-Cu and Al-Zn don't. Al-Mg, Al-MgSi and pure Al have better chances. Cl- is known to botch the alumine layer, independently of the acid. Seawater corrodes most aluminium alloys that way, without acidity. Nitric acid is mild to aluminium because it provides oxygen, hence reconstitutes the alumine layer, and is a weak acid. Pure nitric acid would not attack mild steel neither. But, well, if nitric did attack aluminium, we'd have found some convincing explanation as well... -

Electron injection into a clump of Tungsten, ideas?
Enthalpy replied to Zangetshumody's topic in Chemistry
What do you call "a lot" of extra electrons? Tungsten has only two electrons "above" 5d, so more than two electrons added to 5d couldn't be removed from 6s, and then tungsten would be an ion, which is hard to accumulate. In addition, 5d4 6s2 refers to an isolated atom, not to normal tungsten which has metallic bonds. In heavy elements, the chemical bonds affect deeper shells, not just the most external one. Maybe have an alloy instead of pure tungsten? Several atoms of a very oxidable metal could transfer many electrons to one nearby tungsten ion? Oxidable metals tend to be light and separate from dense ones, so alloying may require the proper element and possibly rapid solidification techniques. Ba, Hf, Ta...? Anyway, this depends on tungsten having a 5d shell in the state you desire. "Clump of tungsten" may be difficult. -
As far as I ignore (which is a lot) no justifiable model exists at all for the proton-proton force. Even the forces within a proton are badly modelled, and not by algebraic solutions. Usual models tell that the force between nucleons works only at contact: they vanish very quickly over distance, and get repulsive quickly if nearer. This explains why all nuclei have nearly the same density. It's a strong contrast with electrostatic repulsion. With such a model, you can forget any solution in terms of orbitals. "At contact" does not allow for a solution as a wave function. Only the force versus distance could, but this dependency is not known. You suggested "slightly attractive", Wiki puts "unstable" and the protons separate quickly in experiments that seem to have observed it http://en.wikipedia.org/wiki/Diproton#Helium-2_.28diproton.29 one might argue that the experiments produced an excited state. Maybe the diproton has a probability to stay stuck that decreases exponentially over time depending on how much energy it lacks (like any tunnel effect), and the improbable transformation into a deuteron depends on the transformation into a neutron within this short lapse. ----- Shall a positron-negaton pair be created to help the fusion of two protons? - This would need additional 1.22MeV if the pair must stay for some time, or until the positron is expelled - There are already negative electrons in a star. About as many as protons. That is, about as dense as in a solid on Earth, and in 7Be http://www.webelements.com/beryllium/isotopes.html the density of the 2s (valence) electrons, or the electron density in a solid, contributes measurably to the frequency of electron capture. - The standard theory seems to be: emission of a positron by one proton, nothing less direct. ----- Van der Waals' equation for dense stars: I suppose others have already answered this, like Chandrasekhar. The pressure of the electron gas must be stronger. http://en.wikipedia.org/wiki/Degenerate_gases#Electron_degeneracy Also, when a star is near to collapsing, its core is no more hydrogen, but heavier elements. Please take with care, I'm uneasy with these questions.
-
The flame of my propane or butane lighter is very transparent to the red light of my laser pointer. No perceivable difference at the laser's spot. Nor do I see any spot from the laser's light on the flame. When I had protected a thin sheet of copper from a blowtorch by having water on the other side, the transferred power density was compatible with thermal radiation from acetylene+oxygen, but maybe the transfer was by contact after all. Or the hotter flame is more opaque.
-
Since planets have been detected around other stars, and life was observed on Earth under conditions previously considered very hostile, the guess of scientist has shifted towards "life on many places in each galaxy". Though, with nearly no significant information to support one answer or an other, the status of this question has barely moved from "pure philosophy" to "this question is scientific but has no answer yet". As for intelligent life, one uncertainty is how long it stays. An other is: how intelligent? An ant? A raven? A chimpanzee? A sapiens sapiens with books? As for distance: the planets we detect are few tens of light-years from Earth. Given the importance of an other civilization, we could wait that long. And maybe information or matter can travel faster, though this fits nowhere in our working theories.