-
Posts
3887 -
Joined
-
Last visited
-
Days Won
1
Content Type
Profiles
Forums
Events
Everything posted by Enthalpy
-
Different collapse processes must be distinguished. Any mass is supposed to form a black hole if it becomes concentrated enough, including dark matter. This is not related with nuclear reactions, formation of neutrons or other. As long as a star radiates enough light, radiation pressure prevents it from getting too dense and collapsing into a black hole, which would also need said star to be heavy enough. Smaller stars that have stopped fusion without collapsing to a black hole can make other compact objects. For instance a neutron star, if they have the proper mass. Because most stars have companion(s) and exchange mass, more varied and complicated scenarios occur. ----- The total mass of a black hole isn't straightforward because gravitation energy changes during the collapse and this energy is mass... Am I right to believe that all mass added to an existing black hole adds fully to the hole AND its equivalent is radiated as electromagnetic waves and particle jets, so that the added mass "counts twice" as it falls in the gravitation well? And what about the initial mass that creates the stellar black hole: is the hole as heavy as the visible matter not expelled that collapsed? How much energy gets radiated during the collapse: any mass exceeding Schwarzschild counts twice? Thanks!
-
It all depends on what one calls "difficult mathematics"... QM is all about waves, and accordingly is as complicated as waves are: optics, acoustics, radiocomms... It does add some formal operators, which are linear algebra. Nothing tragic, even an engineer can hope to grasp it. That's maths created over a century ago (Fourier). Present-day maths are horribly more difficult.
-

Pour your brains here on how to build this contraption...
Enthalpy replied to Externet's topic in Engineering
Shape memory alloys are faster and perhaps a bit smaller than the melting alloy, but apparently their transition doesn't fit between +5°C and +25°C. Only Ni-Ti is available commercially if I searched well, and its transfomation range is for instance +23°C to +35°C in one direction and +6°C to -3°C in the other. The range is shifted by the precise composition, with a 10K additional tolerance, but stays wide; Ni-Ti-Cu would improve by 15K, still too wide. So melting remains the best candidate for the Ocean's temperature gradient. By the way, a pressure that lets harvest work in a cycle must shift the melting temperature significantly. Melting actuators have been used for decades as thermostats. They base on wax, which eases the design so I may have a look, but the stiffness of an alloy lets me suppose it offers more work, and its conductivity allows a wider tube. The melting actuator or motor is good for the glider, say at Ocean research, and generally to exploit small temperature differences like the Oceanic gradient. I don't expect a better ideal efficiency than with a gas or a vapour, but a net power output is easier to obtain. Autonomous mechanical power is sometimes more important than efficiency. For instance to harvest cold water from the depth of the Ocean or a lake, it takes little power to lift the denser water, the temperature difference is available at the engine-pump, and the engine avoids to mingle electricity with water. Once at the shore, cold water can cool houses, say in Canarias, Baleares, Hawaii, the Great Lakes... Marc Schaefer, aka Enthalpy -

Pour your brains here on how to build this contraption...
Enthalpy replied to Externet's topic in Engineering
Fusion of a solid, especially of an alloy for stiffness and fast heat transfer, brings volume at a big potential pressure, and makes the design about as compact as shape memory materials. The 75.5-24.5% Ga-In eutectic melts at +15.7°C - other proportions warmer, but cooler with added Sn. Lacking data, I suppose here 3% expansion and 62kJ/kg latent heat at melting, and both for the solid and liquid, 25W/m/K heat conductivity and 25GPa bulk modulus. A D=10mm cylinder freezes and thaws in just 15s from ambient +5°C and +25°C, and the pressure increase at constant volume would be 7500b, demanding a stiff design. If used at 1500b, the volume change is 3% -0,6% (Ga-In) -0,3% (tube) -0,2% (hydraulic fluid) -0,2% (others) = 1,7% and the available energy 2500kJ/m3, yeah. The same bladder needs only a total of 23m of D=10mm thawing alloy. The thick tube (OD=20mm, ID=10mm) is plated Cu-Be2 while the brazed fins can be Cu-Cr1Zr or cold-drawn Cu. The hydraulic fluid is glycerine with some anti-freeze water, or a PEG-base fluid, hold in a collapsable polymer tube (of shrinkable sleeve?) of D=2mm according to the alloy's melting expansion. A spacer (for instance thin spring wire) leant against the wall holds the polymer tube at the center from place to place, while a small metal tube (not sketched here) inside the polymer tube, with tiny performations, ensures the fluid can pass, and connects to the use. Catalogue static and even mobile joints exist for 1500b but not for 7500b. I'd say: brazed metal tubes everywhere, short and thick, and a ball-and-cone valve. Marc Schaefer, aka Enthalpy -

How can light go back to it's original angle after being refracted?
Enthalpy replied to arknd's topic in Classical Physics
You may imagine photons absorbed and re-emitted if you wish... It's more than an image, it's also a means for numerical predictions - a model, in other words. BUT beware one book has spread a false idea. Re-emission takes no delay. It's the electric polarization of the medium (usually the molecules) reacting to the electric field that makes photons slower. As well, absorption and re-emission don't mean that light is scattered. It can stay perfectly coherent and focussed, for instance in a telescope. Hence stay away from the image of bouncing light. For instance, a gas more compressed slows down light more, though bounces would change the direction by just as much. ----- About energy: you seem to imagine the photon's energy like a kinetic one, and though it's not false, this image can be misleading. The photon's energy is uniquely linked with its frequency, which doesn't change from one medium to an other. -

Pour your brains here on how to build this contraption...
Enthalpy replied to Externet's topic in Engineering
Still for an expanding liquid like oil, this heat exchanger works faster than the set of tubes. At the oil side, I've considered fins 29mm long, 2mm thin and spaced by just 2mm, so the oil temperature follows with only 1.5K lag a water gradient of 20K in 100s. Now the glider is fast enough to swim against a strong stream. The fins at oil and water side are milled by a disk cutter or several disks on one shaft, the tips completely deburred, cleaned and covered with a layer of excellent filler, then an upper and a lower set of fins are interleaved and pressed (vertically on the sketch) to the proper overlapping. The interleaved zone is pressed together (horizontally here) and the filler molten. This needs proper tolerance at fin spacing. The tips must be tapered and filler drops extend only there. Consider etching chemically the tips, for instance with lukewarm FeCl3, which can deburr and, when dipping deeper, adjust finely the interleaving tolerance. Some additional (or all) filler can be flown in from two sides, or preset as a powder between the cold fins above the interleaved zone. The end caps must be really stiff and leave little dead oil volume. If considering elastomer joints, check their compliance. Void and heat help filling without bubbles and outgas the oil. I take Cu-Cr1Zr for the fins, possibly for the caps. It may need additional anticorrosion protection, especially at the joints. If the fins zone is 0.25m wide and 0.8m long, the previous bladder needs 8 such exchangers. The exchangers could be the glider's stabilizer, but if put instead in a hull recess, they drag less and can be protected from dirt. All ends should be tapered against algae and may extend more than the fins. Such an exchanger has uses beyond the oceanic glider. Marc Schaefer, aka Enthalpy -
I mistook it for CaCO3. My bad, sorry.
-
The new owners of the house removed the pot of toluene they had placed, as well as the false book alleging toluene is very toxic?
-
Dielectrics work by reducing the field hence voltage for a give charge, and increase that way the capacitance. They do so by allowing their charges to move a little bit when the field is applied. These charges can be electronic orbitals which deform, they can also be ions. Sometimes the ions can have two stable positions, in which case the material has an intrinsic polarization which the external field can reverse; these materials are called ferroelectric by analogy with ferromagnetism hysteretic behaviour. These are PVDF and the type II and type III ceramic capacitors, very compact but with high losses, microphonic sensitivity, temperature drift and so on. Such a comparison holds only to maximize the capacitance... One may want to maximize the energy instead, and then the allowed electric field is twice as important as the capacitance increase (=the permittivity), especially if a capacitor can be made of layers as plentiful and thin as desired. Then non-polar plastics or vacuum can be about as good as ceramics; it depends on the capacitor's voltage. In our complicated world, the maximum field depends on the voltage. Most users, especially for electronics design, want other properties than compact capacity from their components: temperature stability, time stability, frequency uniformity, low losses, low "dielectric polarization" (charge give back with a delay), low inductance, linearity, self-healing ability... These subtle properties, which would deserve much more attention, tend to demand dielectrics that don't make compact capacitors.
-
NaOH stored in the atmosphere for 5 years? Very much of it has reacted, I'd say. From previous observation, NaOH first absorbs air moisture - at least in temperate climate - to form a paste. If your thing is dry and hard now, it could well be that absolutely all NaOH is converted into Na2CO3 or NaHCO3. Smash some and try to dissolve: Na2CO3 doesn't, NaHCO3 and (mind the heat) NaOH do. Use pH paper, cheap and available, to see if it's still any alkaline. But if you know the initial weight, you could just compare.
-

Tips on writing a research paper? Does anybody have any?
Enthalpy replied to howlingmadpanda's topic in Amateur Science
You mean fusion reactors? They need deuterium (to be separated from hydrogen, this is the main effort, not the obtention from a compound) and tritium (not available on Earth, stopping problem, not solved up to now). In comparison, knowing where mixed 1H and 2H come from is unimportant. And, yes, I wouldn't call "research" a paper on hydrogen-based energy economy. It was a new idea 40 years ago and has been widely described meanwhile. Not quite sure a mass-medium would accept a paper on this topic. -

Smelting 99.9% pure tin ingot at home, need some advice.
Enthalpy replied to Paul Loatman's topic in Applied Chemistry
Intuitively, I'd try pre-heated Pyrex before aluminium, as it must be flatter and smoother. Just put a tin catcher below in case the Pyrex breaks. Because liquid metals are reactive (tin less so, sure) I'd first make a limited trial with olive oil - in case some carbide forms even below the oil's smoke point. But I may be horribly wrong. 10µm is coarse milling. This is the overall flatness; roughness is far better, like 2µm being very rough to the finger and 0.3µm a medium finish. The operation is so quick that you could find a milling machine at some neighbour university or company - or a turning machine if the roughness can be concentric. Presently my preferred material: cake tins made of silicone rubber. Usable at 350°C in air. Very smooth surface, no adherence, easily removed thanks to flexibility. They may need some additional support with the heavy tin. Got 600 hits on eBay.co.uk for: cake tin silicone including flat ones like items 150979423481, 170981202374, 251135007693 (ID = 8"), 380433105724 (9" * 5") and many more. Ask for flatness and smoothness. Other sources as well. Maybe you could melt the tin in the mold directly, if using a cooking oven. It depends on where the tin oxide goes, hopefully it floats. Then tin will have enough time to outgas every bubble from the mould's bottom. -
Calcium oxide is very corrosive to the skin. Strong advantage to iron.
-

Magnet through a copper coil - What if poles rotated 90 degress
Enthalpy replied to jtotheroc's topic in Classical Physics
Essentially zero effect when the magnet is rotated as on the second drawing. Up to the precision of the angle between the magnet's and the coil's orientations, and secondary effects like eddy currents induced in the thickness of the wire. -
Nitric acid may contain little water but as it gets more concentrated, other NxOy compounds appear spontaneously. Phosphoric melts at +42°C, pure one seems to exist. Carboxylic acids?
-

Smelting 99.9% pure tin ingot at home, need some advice.
Enthalpy replied to Paul Loatman's topic in Applied Chemistry
If pure tin behaves like tin-lead eutectic, then it won't stick to aluminium within a reasonable time because of the alumina layer. Same with stainless steel. You can anodize both to thicken the oxide layer. Though, I made unvoluntarily a good weld joint with tin-lead on aluminium. The liquid was hot, stayed in alu for hours, and had some soldering flux. Ceramic would be my first thought. A strong sheet of Ptfe (Teflon) maybe, but it's expensive. Pyrex: just pre-heat it to 230°C in an oven. No more shock then. Other approach: instead of casting tin, machine it. A milling machine gives you at least 0.01mm flatness. Some school near to you that has one? -

Pour your brains here on how to build this contraption...
Enthalpy replied to Externet's topic in Engineering
The variation of Young's modulus with temperature isn't more compact than the thermal expansion of oil. Shape memory alloys and polymer can be more compact than oil, with alloys possibly faster as well if the elements are thin. Transition between +5°C and +25°C needs to be checked. Marc Schaefer, aka Enthalpy -
Our troposphere's "equilibrium" is far from one of thermodynamic sense, that is at uniform temperature. We use to call it "equilibrium", but it's very nearly one where only the atmosphere's bottom is heated by Sunlight and air is free to move by convection and with convective bubbles exchanging no heat as they climb. You can indeed compute a mean temperature and pressure gradient where isentropic enthalpy compensates for gravitation, or Cp=1000J/kg/K compensate 9.806 J/kg/m. Once you have the temperature distribution, let P vary as T high Cp/R per mole. This gives sensible figures. This is a mean gradient. On sunny afternoons, the temperature gradient is stronger and convection triggers in order to lift our gliders, the birds that guide our gliders to the convective bubbles, and also to even our the heat input in the atmosphere. On cold clear nights for instance, the temperature gradient is weaker, we call it an inversion, which effectively blocks vertical movements. Pollution accumulates. The situation is completely different in the stratosphere which has a uniform temperature (-70°C beginning at 12km, both depending on the latitude) and hence no wheather events. Please refer to "standard atmosphere" for instance there http://en.wikipedia.org/wiki/International_Standard_Atmosphere for few tables and more links.
-

Pour your brains here on how to build this contraption...
Enthalpy replied to Externet's topic in Engineering
I imagined launch from a boat only. The glider needs, as you noted, depth as soon as it is freed. -
Wouldn't water+ethanol+gasoline make a colloid? I imagine water droplets surrounded by a monolayer of alcohol in a matrix of gasoline. Provided water isn't too abundent, of course. In Externet's experiment, how to know if the alcohol is still mixed with the gasoline only? I'd expect some to mix with water as well, though isopropylic is less hydrophilic than ethanol or methanol. And what happens on stirring? Does it get translucent like milk?
-

Pour your brains here on how to build this contraption...
Enthalpy replied to Externet's topic in Engineering
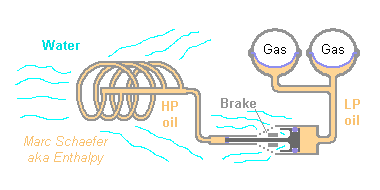
To compress the bladder by a thermal expansion, solids would impose a sturdy unflexible design. Liquids take less room, a design follows. A nearly-perfect gas would be bad; a gas just above the critical point (SF6), a liquid just below (SiF4), a liquid-vapour equilibrium or better a dissolved gas might improve, but I won't check that. http://de.wikibooks.org/wiki/Tabellensammlung_Chemie/_Dichte_gasf%C3%B6rmiger_Stoffe sort by Kritische Temperatur A shape memory alloy, or the change of Young's modulus with temperature, might improve. Later maybe. The liquid here is soybean oil: 746ppm/K and 2.05GPa (near 1atm). Pure biodiesel would be more efficient but leaks more polluting. Better choices must exist. The container and heat exchanger is a set of stainless tubes, ID=6mm OD=8mm, purchased as coil, totalling 1670m. Wound with D=0.6m, it takes 14 layers of 63 turns on 0.76m length. Oil then follows with 2K delay a gradient of 20K in 500s. 0.34dm3 expansion are exploited between 89b and 211b ("impedance matching") on a small piston, say D=46mm, which pushes a big piston, say D=204mm, to control the buoyancy. Steel expansion and elasticty are factored in, plus little margin. Via soybean oil, CO2 is compressed between 80dm3 6b and 70dm3 7.13b, while water counter-pressure varies between 3b and 0b. The gas is isolated from water and has 288K at 75dm3. The controlled brake allows action at both extreme programmed depths, better than hysteresis. Maybe a valve. An oil throat makes for smooth moves. Bladders can be multiple (but mind moving liquid mass), their location modified and the exchanger's one as well... Hydraulic designs are flexible. Please remember that hydraulic and maritime design must be made or helped by experienced people (protect the heat exchanger), and seal design is a specialist's job. The glider's design must boast adjustment for buoyancy (at each campaign), position of the center of mass, position of the bladder(s). The salvage ballast must more than compensate flooded bladders and exchanger plus all compressible parts; it can test the glider without the bladder. The operation team must comprise one person with clear ideas about gas, thermodynamics, buoyancy. Have a tether for each first dive. Marc Schaefer, aka Enthalpy -
Corrosive cyclohexane? Are you sure?
-
If the electron has a good energy, like a beta ray, it has a trajectory in the classical sense. Not a straight one because of shocks. The photo medium's behaviour can be to react only at some points.
-
Capillary action fits tissue paper well. Like: it separate a mix of two liquids. Choose them of different colours.