-
Posts
3887 -
Joined
-
Last visited
-
Days Won
1
Content Type
Profiles
Forums
Events
Everything posted by Enthalpy
-
Levitation melting lifts the melt from the crucible to avoid pollution at high temperature. Patented a century ago, it's often a demonstrator or a research tool, but one team melts 0.5kg for instance to cast titanium impellers for turbochargers 01336015 at hal.archives-ouvertes.fr pdfs.semanticscholar.org and one company melts 500kg 01333975 at hal.archives-ouvertes.fr Most designs are very crude: no magnetic material, hence coils of small section, cooling fluid parallel to the current, wires too wide for the frequency. An expert magnetic designer should improve that. The 0.5kg team claims with citation that an axisymmetric field can't levitate metal at its centre, which has to hold by capillarity. To my understanding, the outlet at the centre prohibits coils there, and this is what reduces the force. A temperature not limited by the crucible would let evaporate less volatile metals. Density would prevent boiling at depth: 10mm of 10000kg/m3 melt add already 1kPa. Possibly metal would evaporate from the lower faces too because the electromagnetic pressure needs a Kelvin effect depth to build up, but any layer of the volatile metal condensed in the magnetic field would evaporate quickly. So I suppose distillation accepts coils up to the centre in a simpler apparatus, shallow and wide. Once the evaporation is finished, the melt can levitate and cool in a lower frequency induction before landing. Many small melts, down to individual drops, could be better than one big to accelerate the evaporation and save electricity. Very small melts could evaporate more quietly, without boiling. Marc Schaefer, aka Enthalpy
-
From Wiki and interpolating: K for K for K for K for K for K for 1Pa 10Pa 100Pa 1kPa 10kPa 100kPa ==================================================== 610 670 750 852 990 1185 Zn 882 997 1097 1412 1660 2027 Pb 1283 1413 1575 1782 2055 Ag 1509 1661 1850 2089 2404 Cu 1497 1657 1855 2107 2438 Sn 1783 1950 2154 2410 2741 Ni ==================================================== Zn is easily evaporated from brass CuZn. At 1356K to melt Cu, Zn has roughly 750kPa and Cu 0.1Pa, clear case with one crucible. Even a few per-cent Pb in brass (664Pa) separate easily from both, optionally in two steps for Pb-Cu. Pb is easily evaporated from Sn63 Pb37. At 1660K for 10kPa Pb, Sn has 10Pa. Leaving a bit over 0.1% impurity in each takes two steps, so crucibles suffice. Ag could be recovered from Sn95.9 Ag3.8 Cu0.7 solder where it makes half the value. At 1782K that give 1kPa Ag vapour pressure, Sn has 43Pa and Cu 44Pa. The pressure ratio 4/100 is also the initial composition ratio, making few steps inefficient. A distillation column is better. Ag could be recovered from Sn61 Pb37 Ag2 solder. At 1660K for 10kPa Pb, Ag has 257Pa so Pb would separate first with very few stages, but then the separation of Ag from Sn needs a distillation column anyway. Cu and Ni can be separated by a distillation column or several crucible steps. This needs high temperatures. Cu and Sn shouldn't be separated that way. With Pb, Ag, Cu, Sn, Ni more noble than Mg and Al, the ceramics MgO and Al2O3 have chances to resist the molten alloys and possibly molten Zn. Suggested operating temperatures in air are 2500K for MgO, 1800-2100K for Al2O3, with big variations. Marc Schaefer, aka Enthalpy Hi JC, thanks for your interest, I'll come back!
-
Hello everybody! Pure silver is too soft and often alloyed to make objects. For instance sterling silver often contains 92.5% Ag, plus Cu, possibly Ni and others. Recycling may need to separate Ag from cheaper alloy elements. I propose to distill Ag away from Cu, Ni and others. The 1atm boiling points spread nicely: Ag 2162°C – Cu 2927°C – Ni 2913°C Reduced pressure would make at least the temperature compatible with ceramics like MgO, ZrO2 and maybe Al2O3. Distillation would take far less energy than electrorefining. One step, without a distillation tower, seems to suffice. Marc Schaefer, aka Enthalpy
-
Even if I completely botched the explanation based on molar volumes, but some melt achieves to dissolve selectively some of the lanthanides from mischmetal powder, I'll be happy of course. The molar volumes could be computed, but this is already done at webelements.com The ratios of molar volumes are 1.4 = 18.3/13.0 for Pb/Li, they mix 1.6 = 16.3/10.2 for Sn/Au and Sn/Ag, dissolve 2.3 = 16.3/7.1 for Sn/Cu, no or little dissolution The situations aren't quite the same because Pb and Li have melting points (601K and 454K) closer to an other, while I wonder about "dissolution" for Au, Ag, Cu which are far below their melting point (around 1300K) in just molten Sn-Pb (456K).
-
Most electrical engineers have seen soldering tips eroded over time. Up to now I believed it resulted from the acidity of the flux. If copper in SnPb improves that, fine! But why don't do they it all the time since Cu is cheaper than Sn, instead of selling "neutral flux"? My point is, if you weld with Sn-Pb once on 5µm Au or Ag, the 5µm are gone immediately. If you weld 7 times on 35µm Cu, the Cu is still there. So Cu is observably less soluble in Sn-Pb, or less quickly, than Ag and Au. Yes, an attempt as tritium regenerator blankets for tokamaks. I rang the bell about the pollution by these blankets. I guess solubility rules address only small solute concentrations. Around 1:1 mole, it's no more a matter of solvent and solute and more of intermetallics. Yes! The sole reason for my ramblings is that I don't have the means to experiment.
-
Eu, Yb, La have a molar volume distinctly bigger than the other lanthanides. Could this serve to scoop them from the mischmetal? cm3 ========= Sr 33.94 Ba 38.16 K 45.94 ========= Er 18.46 ... Ce 20.69 La 22.39 Yb 24.84 Eu 28.97 ========= Ligands make complexes selectively with preferred ion sizes. I bet this is already done for lanthanides. Alternately, I suggest the selective dissolution of Eu, then Yb, later La, in a molten metal, if there is such a selectivity. Still stretching the previous logic, the solvent would be a metal with huge molar volume: Sr, Ba, K and few others, at a temperature where lanthanides are little soluble. Then, Eu would dissolve preferentially from mischmetal powder. With more heat, Yb would be scooped, and even more heat would extract La. Extraction could evaporate the solvent. Moving the mischmetal and the solvent in opposite directions would extract much of the target element but evaporate only well loaded solvent. Maybe. And playing with matches, the big way. Marc Schaefer, aka Enthalpy
-
Ramblings about the solubility of metals in melts. Ag and Au dissolve in molten Sn63Pb37 but Cu doesn't. The difficulty to separate atoms from the solid metal, represented by the vapour pressure, is one factor. The temperature for 1kPa is 1782K for Ag, 2281K for Au, 2089K for Cu, so there are other factors. The strength with which the melt incorporates foreign metal atoms is the other factor. Cu, Ag and Au have similar valences, so this isn't the reason. But the molar volumes can explain these metal solubilities. Nothing revolutionary to metallurgy. cm3 ========= Cu 7.11 Ag 10.27 Au 10.21 ========= Sn 16.29 Pb 18.26 ========= Sn and Pb being much bulkier, they dissolve Ag and Au a bit, and the smaller Cu less. So to build a distillation tower, what metal would not dissolve in molten mischmetal? Refractory metals are small and lanthanides rather big: cm3 ========= Re 8.86 W 9.47 Ta 10.85 ========= Er 18.46 ... La 22.39 Yb 24.84 Eu 28.97 ========= This gives some hope that they dissolve little (or slowly). If following that stretched logic, W would dissolve less than Ta, and expensive Re even less. Marc Schaefer, aka Enthalpy
-
Nb and Ta too are reportedly difficult to separate, so a centrifuge might help too. A mole of Nb weighs 93g, Ta 181g, so TaCl5 is 88g heavier than NbCl5, 30* easier than uranium enrichment. Isotopes let that fluctuate by +-5g. After the centrifuge is built, it can optionally spend some time separating 35Cl from 37Cl to save time separating Nb from Ta. NbCl5 melts at +205°C and boils at +248°C under 1atm, TaCl5 at +216°C and +239°C (decomp). A tube of 2000MPa Maraging steel rotating at 416m/s makes 7.7kJ/mol difference in the kinetic energy. At arbitrary +127°C=400K, that's 2.3RT, so the metals are pure in half a dozen steps, or 1 or 2 steps with tubes of graphite fibres. The oxides mix is reduced, Cl2 or HCl give the mix of pentachlorides, centrifuges separate the pentachlorides before reduction. No fluorine needed. I can't tell whether the process is globally advantageous. Marc Schaefer, aka Enthalpy
-
The more refactive lanthanides would distill under lower pressure to spare the column's materials. Beginning here with terbium, all data from Wiki: 10Pa 100Pa 1kPa mp Element K K K K =================================================== 1979 2201 2505 1629 65 Tb terbium 1973 2227 2571 1208 59 Pr praseodymium 2028 2267 2573 1585 64 Gd gadolinium 2103 2346 2653 1925 71 Lu lutetium 2194 2442 2754 1068 58 Ce cerium 2208 2458 2772 1193 57 La lanthanum =================================================== Tb and Pr spread more at 1kPa, Pr and Gd spread less badly at 10Pa and 100Pa, Ce and La remain close. The process speed must be a limit. Marc Schaefer, aka Enthalpy Hi JC, thanks for your interest! I have data about Nb, Ta, W, Re creep at heat and could have a look. In the low-pressure distillation tower, the trays don't feel the atmospheric pressure. Several thin cylinders of refractory material can enclose the trays as a heat insulation that limits the condensation, enclosed in a strong cylinder that operates at a lower temperature. This may need an additional gas like argon. Marc Schaefer, aka Enthalpy
-
Hello everybody! The chemical separation of rare earth metals is difficult and costs more than the extraction of the ore. So could distillation separate rare earth metals? (Of course I didn't check if it's already done). Most 1kPa boiling points spread nicely. Exceptions are 66K for Tb/Pr (or 2.6%, comparable with 100°C and 110°C), 18K for Ce/La, 2K for Pr/Gd. For those pairs, the 1atm boiling points or the melting points spread, so a later step might separate them, distillation at a different pressure or a chemical process. 1kPa 1atm mp Element K K K ============================================== 1047 1465 1097 70 Yb ytterbium 1234 1796 1099 63 Eu europium 1421 2061 1345 62 Sm samarium 1570 2217 1818 69 Tm thulium 1954 2831 1680 66 Dy dysprosium 2040 2964 1734 67 Ho holmium 2163 3132 1802 68 Er erbium 2296 3336 1297 60 Nd neodymium 2505 3491 1629 65 Tb terbium 2571 3779 1208 59 Pr praseodymium 2573 3535 1585 64 Gd gadolinium 2653 3663 1925 71 Lu lutetium 2754 3705 1068 58 Ce cerium 2772 3726 1193 57 La lanthanum ============================================== The distillation tower's material is badly difficult. The best refractory metals have a very low vapour pressure at such temperature (notice the 1kPa, 1Pa and 13mPa), but I suppose they dissolve in the molten mischmetal to pollute the most refractory rare earth outlet and fail mechanically. 1Pa mp Element K K ============================================== 2639 2128 40 Zr zirconium 2689 2506 72 Hf hafnium 2742 2896 42 Mo molybdenum 2942 2750 41 Nb niobium 3297 3290 73 Ta tantalum 3303 3459 75 Re rhenium 3477 3695 74 W tungsten ============================================== Would plain ceramic, or a ceramic coating, resist molten mischmetal better? For the following oxides, I'd say no. I've taken only oxides of refractive metals because of MgO's bad example: liquid Al corrodes it as metallic Mg is volatile. The heat of formation per mole of oxygen atoms is -409 to -635kJ/mol while rare earth metals have -568 (Eu3O4) to -633 (Er2O3). I haven't checked the carbides nor borides. Graphite? 13mPa dHf/O mp Compound K kJ/mol K ============================================== 2200 -409 2145 Ta2O5 2300 -635 2683 Y2O3 -380 Nb2O5 2500 -550 2973 ZrO2 2800 -572 3031 HfO2 ============================================== -568 Eu3O4 -633 Er2O3 ============================================== So maybe uncoated C, Ta, Re, W can make the tower to distill the more volatile rare earth metals, which might be useful. Marc Schaefer, aka Enthalpy
-
RSP Technology uses rapid solidification and sintering to produce alloys with unusual compositions and properties. They have improved and widened their product sprectrum Precision equipment at RSP Technology A frame with 11.6ppm/K from their RSA-453 (Al-Si50) would match carbon steel strings perfectly, better than cast iron does. E=110GPa for 2500kg/m3 are 1.67* better than steel and cast iron, as good as TiAl, wow. Many hypothetical string materials need nearly the same frame expansion as carbon steel: Maraging, martensitic stainless (optionally with precipitation hardening). Strings of CoCr20Ni16Mo7, Duplex stainless or nickel superalloys would be stabilized by 13.6ppm/K from the RSA-443 (Al-Si40) and RSA-441. Strings of austenitic stainless (optionally with precipitation hardening) would be stabilized by 17.3ppm/K from the RSA-4019 (Al-Si20 etc) and RSA-461. The elongation at break is worse than brass. The parts I made of RSA-708 didn't break by falling on the floor and could withstand significant deformation by hammer. Last time my employer needed an ultra-strong rod from RSP, he just paid and got it. Some 30€/kg are a hurdle for music instruments. I haven't seen tubes nor exotic profiles from them, so a frame should use rods, or waste costly material, or convince RSP to experiment. Marc Schaefer, aka Enthalpy
-
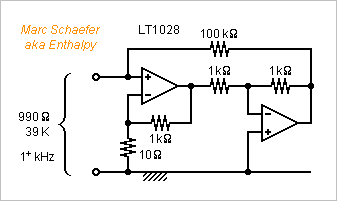
Now with a diagram : The LT1028 produces beyond 1kHz typically 0.85nV/sqrt(Hz), and as seen previously, its 1pA/sqrt(Hz) convert to 1nV/sqrt(Hz) in the synthesized 990ohm, and the 100kohm add 0.4nV/sqrt(Hz) only. 9.9ohm in the feedback add 0.4nV/sqrt(Hz). This sums to 1.43nV/sqrt(Hz), as much as a 990ohm resistor at 39K. Obtaining all the gain from the first stage preserves the loop's phase margin and makes the second stage's noise negligible. Marc Schaefer, aka Enthalpy
-
I checked on eBay the price of the HP-48G, and ¡caramba! All newer calculators must be bad since users still want this oldie. So I checked the price of my even older defunct HP-15C and it's even worse, ouch. One company swissmicros.com grasped that and makes an Helvète-Packard copy called DM-15L which sells new and improved for cheaper than old used HP-15C, but it costs something. Free HP-15C emulators run on a PC. Good alternative, especially if you're used to the physical one. HP did offer the emulators for free, a cache is there calculatrices-hp.com over web.archive.org The HP-50G computes complex trigonometric and hyperbolic functions. All hide in submenus even deeper than the HP-48G, so I can't recommend it. The HP-15C does the job. But it has no usable keyboard shortcuts, and you have to click first on the f and g prefixes, so I wouldn't recommend it. But the installer brings the handbooks! Do not take this other HP-15C emulator, it ignores complex numbers: hp15c.com Torsten Manz made the best I've seen, downloaded from there heise.de Its blue and yellow tags around the keys are clickable, it reacts to the PC keyboard, and it's multilanguage. Tried briefly, it does complex acos and acosh as needed for Chebyshev type I and II filters. Nice! Ah, to exit the complex mode, just click g > CF > 8 to clear the flag 8. You guessed.
-
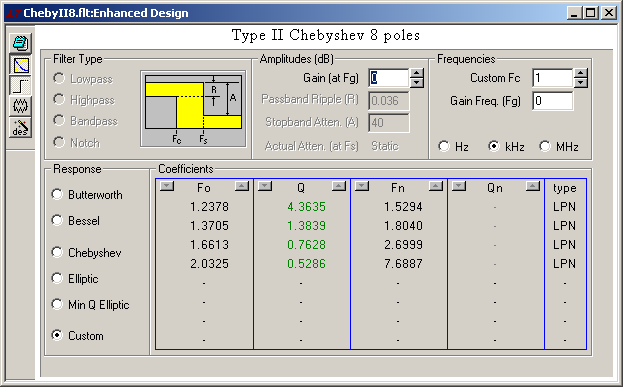
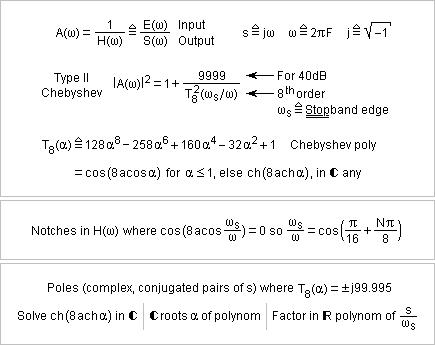
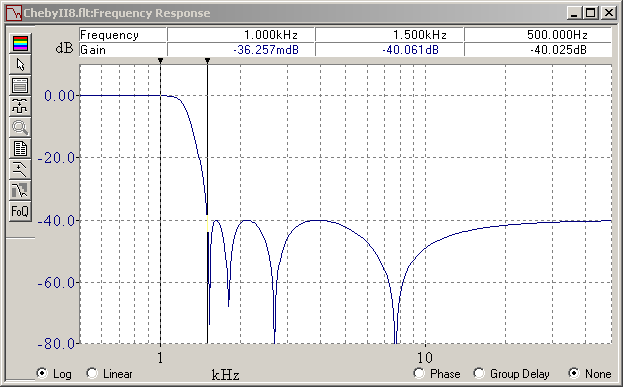
Here's a type II (or inverse) Chebyshev that fulfils the same 0.1dB in the passband and 40dB in the stopband starting at a frequency 1.5* higher. FilterCad 3.0 doesn't design type II Chebyshev automatically (and its Min Q Elliptic falls short of the wanted frequency response). NuHertz does but isn't free any more beyond 3 poles. The design takes longer. The pocket calculator HP48G solves the complex ch() and ach() easily, as it does with cos() and acos() for type I Chebychev, but mine just died. Mathcad 2000 is rumoured to factor real polynoms as first and second order terms, to be reinterpreted here as (w2+ww0/Q+w02) but I didn't find how. But Mathcad did give the complex roots of T8(alpha)=+-j99.995. Injecting the notches and poles in FilterCad gives the adequate frequency response. The step response is disappointing. Last time I compared, the type II Chebyshev was clearly quieter than the elliptic. Possibly I had a stronger attenuation and more room between the pass- and stopbands. Here some poles of the type II Chebyshev are in the stopband, so obviously it works outside its comfort zone. Marc Schaefer, aka Enthalpy
-
And? The chromosphere is thick enough to be opaque, so we see only the chromosphere, which radiates like a black body at its temperature. Nothing to change in my previous post. The line absorption spectrum needs atoms cooler than the blackbody radiation and between the emission depth and us. Hence "temperature varying with the depth".
-
Hybridisation is wrong in QM. I suggest you to check present-day theory, which is molecular orbitals. For instance in methane, there is no sp3 orbital between the carbon and one hydrogen. One molecular orbital, resulting from 2s, extends with the same sign to all four hydrogen atoms, while each of the three 2p orbitals reaches two hydrogens in one lobe and the other two in the other lobe. Spectroscopic measurements confirm two different energies in the molecular orbitals, numerically consistent one from 2s and the other from the three 2p. Hybridisation, with four sp3 hybrids, fails.
-
The narrower wavefunction as exiting the slit allows the electron to be detected in directions that were inaccessible to it when the wavefunction was wider hence more directional before the slit.
-

Do we add fields or intensities in the double-slit experiment?
Enthalpy replied to aknight's topic in Quantum Theory
There are several inaccuracies in the first post. Among others: eikx is for a propagating wave, not a standing one. The amplitude psi does not decay with distance r as 1/r after exiting a slit. This would be for a point or spherical source. In the zone useful for interference, the frontwave area increases like a cylindrical area does, and so does psi2 decrease, because it varies like a power density, since a photons is thought like an energy quantum. Beware with the idea of local detection ! This is badly difficult to understand in QM, most book convey a wrong interpretation. The simultaneous histories of a photon don't vanish upon detection. The double slit experiment is misleading in this aspect. You could check different experiments, for instance interferences of atoms that both have or have not absorbed a first photon and then can absorb a second one: the observed atom interferences tell that after the first light ray, the atom is in both states, having absorbed a photon and not. Consequently, said photon too is on both states, having been absorbed and not. More generally, starting QM with the double slit is a very bad idea. Starting with the wave function of pentacene observed by atomic force microscopy would be better. It would show that the wave function is observed, and that the same pair of electrons is observed over the whole molecule size without any destruction. Causality and propagation delay: no huge difficulty. At least two answers cope with it: - External objects, including humans, have no influence. Then the simultaneity does not transport information, because no information can be encoded. Faster than light is then possible. - There has been no collapse at all of the wavefunction. This is the preferred explanation now, especially in light of newer experiments like the eraser. All possible events did happen, so no information transfer is necessary. What looks like a collapse is only that the observer, if he is in the state having made the observation A, does not feel its states having made the observations B because there are so many and uncorrelated that they sum up to zero. Negative probabilities aren't needed. As Swansont said, |psi|2 is computed after summing all necessary psi. This is what lets waves interfere and tells us that photons or electrons are waves. Beware with the destruction of interferences by knowing "which slit". Meanwhile, "weak measurements" have been experimented, and "knowing" isn't binary. That two apertures half as wide work as one of full width is Huygen's principle. And, yes, diffraction exists with single apertures too, for instance optical lenses. The computation method, summing over all possible positions, is the standard one. sinc just results from summing a uniform illumination over a window width. You can just forget the dimension along the slit for that and compute 1D. Write the phase shift along the width of the slit due to the considered propagation direction, you get the sinc. "Zero momentum information" from a point source is wrong. If the particle has a spin, for instance the photon has, then the directions perpendicular to the (emitted or selectively detected) straight spin are more strongly illuminated (stronger psi2 blah blah blah) while the direction aligned with the spin aren't at all, and in between the pattern is a cosine. I'll stop here for lack of time, apologies, even before having gotten a general sense of the thesis. Strong encouragements to go on thinking by yourself, because most explanations about QM are badly wrong. Most books just reproduce the misunderstandings of the very early times of QM, before decisive experiments were done. Only personal thinking can debug that, and is consumes horribly much time, alas. My suggestion is to consider soon other experiments than the double slit, among them, images of the pentacene by atomic force microscope (search words). -
To bring heavy equipment to an asteroid, including in the main belt: https://www.scienceforums.net/topic/76627-solar-thermal-rocket/?do=findComment&comment=1014780 To explore the main belt and check if anything is worthy there: https://www.scienceforums.net/topic/76627-solar-thermal-rocket/?do=findComment&comment=1016237 Braking in Earth's atmosphere is for free. From low-Earth-orbit, deorbiting costs less than 100m/s. From the asteroid belt it's much more. What we might find there, I have no idea. The general hope is that precious metals have not sunken there as they did on Earth and would be more accessible. This would need to separate them from worthless Ni and Fe on the asteroid, and I have no idea how to do that. It needs very heavy equipment to extract very little precious metal. Human activity could absorb much more gold than we produce, but not at the present price. Best material for electric contacts, for corrosion protection, for cleanliness, and so on and so forth. It could replace copper if abundant enough.
-
It's a matter of opacity too (and of reflectance). Stars are opaque because they're big. If they can absorb any incoming wavelength, they can radiate it perfectly too, hence like a blackbody does. The temperature varying with the depth makes this more complicated. More reasons to emit or absorb other wavelengths than the atom's discrete spectrum: In a solid, electrons are shared among many atoms. They get many new energy levels. In a metal, the levels are extremely close to an other. A transition line is fine only if the emission is slow enough. Transitions have their own duration, which is usually shortened by collisions, in a gas, plasma, liquid.
-

Trouble understanding about Circuit Ground ?
Enthalpy replied to usmansa1's topic in Modern and Theoretical Physics
Force from a pole, and a potential, would be the case from an electric field, not a magnetic one. Magnetic fields do act on charges, if there is movement. -

excitation of electron in an impurity level.
Enthalpy replied to souffler's topic in Modern and Theoretical Physics
Hi Souffler, a photon can and does take electrons from the depth of the valence band too, and send them deep in the conduction band. That's why materials absorb many different wavelength, not just light with energy equalling the bandgap. In silicon (indirect gap) this process is much more efficient: you can check that photons with just the bandgap energy are little absorbed. Other bands can contribute the photon absorption too, or secondary extrema in the bands. Recombination occurs most often among the band edges because this is where there are candidates, at least near thermal equilibrium. But under strong injection, hotter electrons and holes (=empty energy levels) can be available for recombination. This happens at laser diodes, whose wavelength is imperfectly defined. The spin and momentum conservation impose other restriction that may need the help of a phonon, making the process less probable. Impurities are isolated in the crystal if the doping isn't too heavy, and then the doping level is nearly a single energy. Excited states can exist, but they differ by few meV, often neglected. These impurity levels do lose and get electrons to and from both bands, and not only at the edges. Choosing the dopant hence its energy level defines the colour of GaP leds. In both bands, the electrons are largely delocalized. If they are states of perfectly defined energy or momentum, they are delocalized to the whole crystal or doping zone. So overlapping is trivial. In dopant levels, the electrons are localized to the atom, so it can jump to a band, but jumping to an other dopant is difficult. -
This can be fluorescence or diffusion for instance. It can work as well when the pumping photon has the energy of the emitted one. Whether one wants to call it the "same" photon, I don't care. If the incoming photon has a decently known direction and the diffused one a direction different enough, we know diffusion has take place. If only one atom is present, for instance one dopant in a crystal (this is done for quantum cryptography) then we know the diffused photon comes from that atom. At the beginning, the photon is very well localized, to the size of an atom, which is much smaller than a photon wavelength. How fast the wavefunction spread results from its equations. An atom is very little directional for being small, so it emits light very broadly. As for time, at least a few quarterwaves away from the small source, the speed of light in the medium applies. In local field, I don't know - looks like EM waves are faster in local field.
-
If the electron has passed the slit, its wavefunction at the exit is as narrow as the slit.
-
The very idea of movement is more diverse in QM. When the electron is an orbital, it's by definition a "stationary" function, whose amplitude doesn't depend on time. You can say that the electron is "immobile" if you wish. That's why it doesn't radiate - QM answered this problem that classical physics had. The "immobile" electron still keeps a kinetic energy, though, because the wave has a limited size, and it can have an orbital momentum and orbital magnetic moment if the phase of the wavefunction evolves with the position around the nucleus. In that sense, QM gives the electron only some attributes of the movement. We could deduce some sort of mean electron speed for a stationary electron, for instance from the orbital momentum, some mean radius, and a mass. Or from the kinetic energy and the rest mass. Usually it's not done, and it would depend on the definition. As opposed, the energy and the orbital momentum are well-defined in an orbital. Linear combination are solutions of the electron's equation too. If you combine several orbitals of same energy, like 2px and 2py, you get a stationary solution which is an orbital too. If you take orbitals of different energy like 2p and 1s, the linear combination is no more stationary. It's still a wavefunction but not an orbital. The combination has a (or several) bulge that moves over time, at a frequency equal (to a factor) to the difference of energies. Now you have a movement, in the classical sense too, and the electron emits or absorbs a photon. This movement is as fast as the frequency of light, for instance 5*1014Hz.