-
Posts
3887 -
Joined
-
Last visited
-
Days Won
1
Content Type
Profiles
Forums
Events
Everything posted by Enthalpy
-
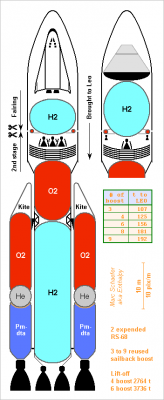
This shall launch the manned Mars mission I describe there http://www.scienceforums.net/topic/83289-manned-mars-mission/ with performance flexibility, and cheaply as it expends only two RS-68 and reuses its sailback boosters. It's as bulky as a Shuttle but with more side boosters and a fairing on top. Click for full size. The second stage weighs 43.7t empty plus 709t or propellants, its 3.4m wide RS-68 bring 4204m/s=429s and 3.53MN. Each booster weighs 50.7t empty and expands 419.4t of oxygen and Pmdeta from 36bar (ending at 19bar for 2.8g) to 0.56bar in D=4.4m for 2922m/s=298s @vac and 9.3MN, 2509m/s=256s @sl. SLS160.zip 8 positions permit 4, 6 or 8 booster, while 9 permit 3, 6 or 9. I've only evaluated simultaneous boosters; six wouldn't push well two inert ones at the end, but throttling two early to keep them longer, possibly while the second stage pushes, looks interesting. ---------- Most second stage's walls consist of aluminium extrusion as I describe there http://www.scienceforums.net/topic/60359-extruded-rocket-structure/ 5690kg D=11m fairing, AA6005A, 45° 1mm 1mm. Thinner would improve. 2397kg D=11m adapter, AA6005A, 60° 1mm 1mm B=38mm. The Leo payload rests on top. 1749kg D=8 to 11m cone, AA6082, 60° 1.5mm 1.5mm B=53mm. 380kg top oxygen elliptic head, AA7022, 1.7mm. 2120kg upper 3.6m of oxygen tank, AA7022, 45° 2.8mm 2mm. 1855kg lower 2.7m of oxygen tank, AA7022, 45° 3.5mm 2mm. 1258kg bottom oxygen spherical head, AA7022, 4.5mm. 169kg foam 4800kg intertank truss. 16 nodes (if 4 6 8 boosters) and 2+2 levels. Heat-treated Ti-Al6V4 tubes, upwards OD=141.4mm ID=130mm. Downwards lighter, stiffeners inwards also. Is it oversized? 380kg top hydrogen elliptic head, AA7022, 1.7mm. 11980kg 25.1m hydrogen tank, AA7022, 45° 2.0mm 2mm. 544kg bottom hydrogen elliptic head, AA7022, 2.4mm. 395kg foam 400kg engines truss 13800kg two wider RS-68 300kg command and control 1000kg undetailed The structural tanks weigh 39kg/t of propellants, the empty stage 29.7t or 61kg/t. I didn't compare with a common head and boosters pushing a truss between the oxygen tank and the payload. The sailback boosters are of welded maraging as usual, they weigh 121kg/t of propellants thanks to throttling. ---------- Inert masses are heavy here. Three stages instead, the third's RS-68 with a 6m extensible nozzle, the second's three RS-68 3.4m wide, would weigh only 2760t to put 160t into Leo - but cost probably more. A corresponding drawing may follow. Marc Schaefer, aka Enthalpy
-
At normal temperature, isolated ions don't form in significant amount. They attract electrons to neutralize. In a polar solvent, especially water, ions can separate from the carriers of the opposite charge. Thanks to its polarization, water permits the cations and anions to coexist. Though, it needs willing anions: magnesium chloride is soluble, the oxide isn't. Two electrons because this is magnesium's valence. With simple atoms (not transition metals), the outermost shell participates in chemical reactions, the deeper ones don't. Well, that would be if chemistry were simple; for instance xenon can make compounds despite its complete outer shell. Also, many elements have several valences, like 2 and 4 for carbon. ----- I wrote "I wonder whether it makes sense to tell if the electrons have passed from one atom to the other" and yes, it makes sense. Anions and cations in a solid use to touch an other so that one can't tell whether the electrons in between pertain to one or the other atom, but it makes a difference nearer to the nuclei. And in a solid, anions and cations do carry a charge, as ceramic capacitors show. The displacement of Ba2+ or Zr2+ between two positions in (Ba, Zr)TiO3 induces a charge flow at the capacitor's terminals, so Ba and Zr don't displace as many electrons as protons.
-

Question for a petroleum chemist/engineer.....
Enthalpy replied to pippo's topic in Organic Chemistry
Even with simple and similar compounds, viscosity wouldn't combine as a mixing proportion. Some formulae exist, like combining the logarithms as the mixing proportion. With very different viscosities, the blend is thinner than combining linearly. But with 10w and 20w, this difference is negligible. -40 implies additives to control this viscosity index, so the viscosity drops less at heat. Things like polyglycols are used. If the two oils use very different additives, there might be surprises. And of course, you have incompatible lubricants or hydraulic fluids, which may even not mix at all, like oil distillates and water-based hydraulic fluids. There are phosphates, polyglycols and several more families, very different from petroleum. -
To my limited knowledge, H+ serves every day in reactions with ethylenic compounds which are essentially nonpolar. This reaction makes much of gasoline, in refineries' alkylation units. Magnesium hydroxide is very little soluble, but if we take NaOH as an alternative example, Na+ is just a simplified writing. As you noted, Na+ won't stand isolated because it attracts electrons and OH-. In a polar solvent, especially water, writing Na+ and OH- only means that they can be separated by water molecules. If the water molecules are properly oriented and make a chain between both ions, there is nearly no bare charge in the solution. A little bit more subtle: each ion has several neighbour water molecules, and these molecules aren't as strongly polarized to have one electron less at one side and one more at the other. Both effects can about compensate. In addition, every Na+ has many OH- almost-neighbours, so instead of a chain to one particular ion, it's more a a surrounding by neutralizing ions. But the net effect of polarized and oriented solvent molecules is that the ion charge isn't bare; the vicinity of solvent molecules poles reduces the local charge much and stabilizes the ion. In an "ionic" crystal, say NaCl, each Na+ is surrounded immediately by many Cl-, so that any charge acts over a tiny distance, and the energy of the electric field is small. Here neither, ions are isolated - a situation that would have demanded much energy as you noticed. In a crystal, ions and their orbitals touch an other, so that I even wonder whether it makes sense to tell if the electrons have passed from one atom to the other. Let's see what chemists say.
-
rituparna, did you really mean e/m for protons, or for ions? For ions it would depend on the proportion of neutrons versus protons.
-
Hi Walter M, welcome here! As far as I know, every method to prepare deuterium begins by enrichment of heavy water, then reduction to hydrogen, and at last anrichment of deuterium. Because hydrogen reduction demands much energy, it's done as late as possible, when water contains already much deuterium. Electrolysis is one convenient means. Hydrogen diffusion then separates the isotopes efficiently thanks to the big mass ratio. Electrolysis itself doesn't seem to contribute much to the separation. It must be about as efficient as one single step of gaseous diffusion, but it will reduce protium (1H) first, which is exactly the undesired effect since enrichment's goal is deuterium (2H). The reason being that the lighter protium is more mobile than deuterium. I've read conflicting reports about it, but even deuterium production in Norway for Germany during WWII was the reduction step, not intended for enrichment, and was not the only step in enrichment. Enrichment by electrolysis only would need to - Electrolyse all the natural water, instead of the enriched fraction of it; - Repeat it many times with successive concentrations, despite gaseous diffusion does the rest as efficiently and at a low energetic cost, once hydrogen is reduced. So while some separation occurs, it's the wrong direction, and far worse than competing means.
-
And all these neutrinos are produced at the core as I already said, in an amount propotional to these from 7Be, independently of solar storms and flares. How the heck should effects at Sun's surface change the neutrino flux to influence radioactivity on Earth? No, I won't make any further check on that. The curve interpretation in the original paper is flawed. The mechanism shows no relationship with neutrino emission. There would be many thousands of claims to be checked, less unrealistic than this one.
-
Interesting! According to Wiki (thanks Greg H) the oxygen declined but the dioxide didn't increase as much because some concrete soil trapped it. And possibly, organics present initially in the soil to feed the growing plants used excessive oxygen as they were used by microbes instead. From the Biosphere 2 observation, the answer to "how hard would it be" is just "we don't know how to do it". Regenerate oxygen from dioxide is already done in space stations, the input being sunlight, then goinf through things like scrubbers, electricity and more. For instance a process uses a hot ceramic to electrolyze the dioxide. Recycle water is already done as well. I've suggested elsewhere to produce very simple food from waste water and dioxide by chemical means: glycerol is one candidate - but you can't feed a human only on that, it's just a sugar equivalent. Storable food doesn't look very complicated. Seal, sterilize by radioactivity. Some extraterrestrial soil has already proved to sustain plant growth. I don't remember if it was lunar soil brought back here, or terrestrial soil of the same composition as measured on Mars and sterilized - but anyway, plants accepted it. If you need hundreds or thousands of tons of water, consider bringing an icy asteroid where needed, as I suggest there http://www.scienceforums.net/topic/76627-solar-thermal-rocket/page-2#entry757109 updated http://www.scienceforums.net/topic/76627-solar-thermal-rocket/page-2#entry757663 and anyway, the poles of Mars have water ice. Mercury as well, they say. Or grow plants on the asteroid where it is; do lichens grow on solid ice? On Mars, the situation would differ a lot, because oxygen and dioxide can be an open cycle. Only water must be 100% recycled if not at the poles. The real challenge in a closed vessel is to (1) recycle all the waste (2) produce fresh food - which goes together. I believe nobody has a justified solution to that. What we do on Earth has proven to fail in Biosphere 2; in an isolated space vessel, getting cockroaches instead of tomatoes wouldn't be surviveable. Maybe it demands microorganisms that we ignore, or impossible quantities just to guarantee enough diversity. If we sterilize everything to avoid unwanted species, we may well suppress unidentified vital ones. Only further experimentation can tell. It doesn't need to be in space. Biosphere 3 and followers are the way to my opinion.
-
The reaction won't keep both H+ and OH- because they react to form H2O.. This is what happens in a neutralization - and nearly nothing else with strong acids and bases as in your example. The product of H+ and OH- concentrations in water is around 10-14/L at room temperature (somewhat more because the neutralization heats the water) so both around 0,2M is impossible.
-
Wave functions don't require much imagination any more because now you see them: http://physicsworld.com/cws/article/news/2009/aug/27/molecules-revealed-in-all-their-glory-by-microscope http://journals.aps.org/prl/abstract/10.1103/PhysRevLett.94.026803 (click two last ipctures) http://io9.com/the-very-first-image-of-a-hydrogen-bond-1426759827 One funny idea about the atomic force microscope, as opposed to the scanning tunnel microscope, is that you observe the same electron pair all the time. This is not a statistic over many successive electrons. It helps to understand whether an interaction between two particles needs to reduce them to points or not, and more interesting thoughts.
-

Questions about light (split from does light actually travel)
Enthalpy replied to Kramer's topic in Quantum Theory
The photoelectric effect is properly explained by light being absorbed in quanta. Whether it travels in quanta is a distinct question. Light propagates as a wave. In vacuum and with a beam broad enough, this resembles a straight path. In a semiconductor, we observe directly that electrons are knocked away from the valence band, so they're not created. That's consistent with more general rules that charge is conserved, and then an electron+positron pair would need 1022keV, far more than visible light brings. -
No other biosphere than Earth has ever worked in the long term. The latest attempt I heard of, Biosphere 2, stopped after few seasons because carbon dioxide was out of control. Obviously we know too little about life cycle to design a working biosphere. Crop growth efficiency is known, the rest is not. How diverse must an ecosystem be to be sustainable even for few years? Unclear. And what's the minimum size to guarantee food despite natural variability of harvests? The other aspect is that for few years, it's better to bring the food with the astronauts. In open loop, take 8kg per person a day; recycling air and water reduces it to 2-3kg. 2t soil per astronaut would be very little, but it takes already 3 years to justify.
-
And? You would like neutrinos of lower energy to be produced in any significant amount, as compared to those from 7Be and 8B which result from the very proton-proton cycle at the core? I'm perfectly confident that this would be noticed from long. Neutrinos are emitted in decays that produce energy, that would have been noticed. Better: light nuclides and their decays are all known. Also, you'd like neutrinos of lower energy to trigger radioactiv decay more efficiently than those from the proton-proton cycle? That's becoming unrealistic... The reasonable path is that the core emits nearly all neutrinos, including the ones capable of triggering reactions, that these neutrino flux is independent of solar flares and storms, which dont' influence decay on Earth - as the original paper shows, if one reads the curve objectively. Patents prove that a clerk accepted the text. In the US, he must be convinced that the invention works, with all associated uncertainties, in Europe not even. In France at a time when inventions had to look feasible to be patented, a clerk refused the electric transformer. A patent is not a proof of valid concept.
-
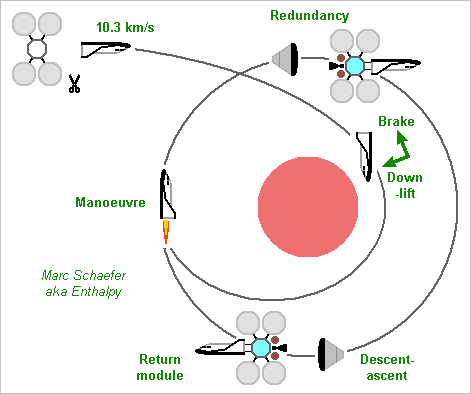
Same as in my previous message, but with colour: crew's transfer from Earth to Mars, with aerobraking. The sketches follow no scale at all, nor are all solar engines represented. Speeds are asymptotic and relative to each planet. Good downlift by the wing keeps the glider in Mars' atmosphere long enough for a bearable deceleration. The same orbit inclination for the descent-ascent module and the return module is useful, to cumulate the solar engines, or if rats have eaten the biscuits at one vessel; then the inclination is ecliptic or nearly, which restricts the landing sites to -25 to +25° latitude with some plains available. This script needs no accurate landing site. It also permits redundancy, with two descent-ascent modules that can help the crew in orbit or on the surface, and two return modules. 180° phasing in orbit doubles the docking opportunities, saving time. This redundancy needs five launches for the first mission only, dropping to three for the following ones. Marc Schaefer, aka Enthalpy
-

Is Relativity 100% proven to all professional scientists satisfaction?
Enthalpy replied to Hazel M's topic in Relativity
Relativity is already an old theory. To me, its correctness isn't any more a question for a handful of scientists. It results from thousands of engineers who use it daily with success. -
About satellites: I've built my own, and more importantly, discussed with SSTL colleagues who had built a dozen and operated them for decades. The result is that (1) Latchup doesn't happen. I had protected electronics against it, they had already suppressed their protections. (2) Lost bits do happen from time to time. That's a good reason to have error-correcting codes, and software with internal checkings and restart capability. The rest is literature, either from the time of >10µm CMOS, or by people whose job relies on circuit protection. Already 4µm CMOS was incapable of latchup. Some bits are lost, fine; this happens on Earth as well, for instance big computers must protect themselves against and do it. Once the hard and software is robust, whether more or less bits are lost during a solar event is unimportant. For power lines on Earth, adequate protection is the solution. Because passive protection is better than prediction. And because we have the proper components now (SiC didn't exist in 1989, vacuum switches were rare), which let the line conductors themselves dissipate the excess energy. Which doesn't prevent people to make more research on the topic and publish it. I've nothing against, but these two justifications are outdated.
-

Theories on possibilities may have ruined my life
Enthalpy replied to Tailspin's topic in Mathematics
The novel that describes a librabry containing all possible books has already been written... The novel's title is "La biblioteca de Babel", in the section "El jardín de senderos que se bifurcan" of the book "Ficciones", by Jorge Luís Borges. Very nice literature. The English translation may have kept "Garden of Forking Paths" separate. http://en.wikipedia.org/wiki/Jorge_Luis_Borges_bibliography it's anterior (1941) to Shannon's theory of information (1948). Though, one should understand that making an art work among infinite possibilities or among a number so huge that it's not exploreable nor even conceiveable is just equally good. It makes no difference for every purpose. It's creation. -
And these neutrino detectors have shown no surge during solar flares nor storms. As expected, since the neutrinos originate at the nuclear fusion reactions deep in the Sun, not at the shallow surface. That's one bizarre claim in the original paper, the other being that one sees variations in the radioactive decay curve without solar activity, and solar activity without effect on the radioactive decay.
-
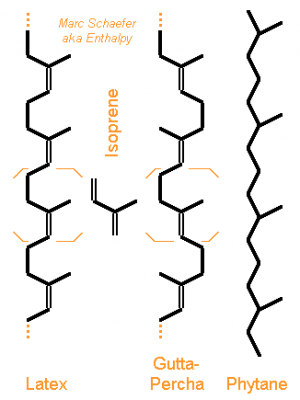
Fantastic! Love the dandelions idea. If some route is practical to obtain the head-to-tail trimer or tetramer of isoprene, then the branched alkanes resulting from full hydrogenation (farnesane, phytane) look great as: vacuum oil and grease cooling liquid for electronic equipment transformer oil and as rocket fuel and jet fuel if cheap enough. Their liquid range is like -100°C to +260 or +300°C, wow. Previous thoughts http://www.chemicalforums.com/index.php?topic=56069.msg202845#msg202845 Extract isoprene from air: circulate the greenhouse's air through a solvent mist? Or to save power, make the air-to-solvent contact like in a humidifier (The drawing was meant for a multiphase reactor, just forget the closed vessel here)
-
Hi externet! Because calcium metal reacts with water, extraction by electrolysis can't be made in water. You would have to dry the salt mixture first, then possibly electrolyze the melt. In seawater, sodium is more abundent, and may be more interesting than cacium. Calcium compounds are usually obtained from limestone instead. You want to produce hydrogen that way? Several attempts exist to store a metal and produce hydrogen when and where needed to feed fuel cells. One is aluminium, doped with gallium to promote corrosion. Though, I expect it's better to use the metal in an electrochemical cell directly, rather than convert it to hydrogen, where potential is lost as heat. Calcium weigh 40g for two electrons, that would be equivalent to 2g of hydrogen. Magnesium looks a bit better, lithium is too scarce for some uses. The big question is: how light (and safe and convenient!) can be the storage of hydrogen.
-
on ebay.com, search Potassium chloride 70 hits there. Though, I've no idea how good each seller and compound is on eCreek. For computer parts or bikes, it's extremely variable.
-
Our atmosphere protects us from cosmic rays. The geomagnetic field rather protects the atmosphere against erosion by incoming particles. Power grids may have been disrupted once many decades ago in northern America through such a process - or not. The proper answer is to build protections in. It hasn't happened again. Same for satellites. Recent electronic components are more resistent, and satellite architecture shields them better. Then, you can design the circuits to survive occasional events. This worry is very largely overvalued: (1) It was serious at the beginning of space exploration (2) It permits to explain satellite failures through bad luck, not designer fault (3) This story serves as an excuse understandable by the public to study our Sun's activity (4) Meanwhile, which components are sensitive or not is known, and the consequences as well, so designers adapt.
-
Sensei, you believe what you want, and even that Solar neutrinos influence radioactivity if you wish... Read again the paper, look at the curves, observe that the correlation with flares is not visible. Consider that solar flares don't change essentially the neutrino flux from our Sun. That the paper wants to see an effect on some alpha emitters - just like 238Pu is. Read the other paper about the RTG: your objections are already addressed there, of course. That an annual variation has billions of possible explanations, the distance to Sun being just one. And, yes, the authors re-analyzed data that was then already 20 years old. Maybe you wish to believe in that paper, but it's bad science.
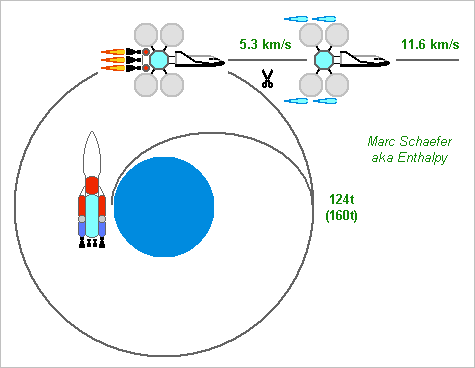
-
----- Crew's leg to Mars, with aerobraking ----- For an 80-days trip not including the acceleration overcost, the vessel moves away from Earth with asymptotic 80° and 11575m/s, and arrives at Mars with asymptotic 10308m/s, or 11468m/s versus a non-rotating ground. The vessel with crew and life support is to weigh 20t at Mars; 500m/s of tri(methylaza)tridecane and oxygen for orbit corrections and rendezvous with the preset descent-ascent module bring it to 24t. The vessel brakes by Mars' atmosphere only; it's a glider to provide 35m/s2 downlift. A 6m2 wing would suffice at 20km height, so higher is possible - the Martian atmosphere is very variable, but the preset return and descent-ascent modules observe it, and the wing copes with some variations. The solar engine accelerates near Earth from asymptotic 5283m/s to 11575m/s by expelling 17t hydrogen, beginning at 42t. Twenty 10m concentrators achieve it in 10 days. This propulsion is thrown away before aerobraking. A chemical engine brings the composite to the 5283m/s above Earth gravity, more than optimum 4283m/s to save concentrators. Four RL10B (optionally with common turbopumps and actuators) burn 76t propellants in 800s. This expended stage starts at 400km Low-Earth-Orbit with 124t, so the vessel could be bigger. I wish to unfold and test the Solar engine before sending the crew away from Earth. The unfolded engines must then withstand 1G, which also permits to test on Earth. Integration isn't obvious. A sketch of the transfer should follow. ----- Variants ----- Aerobraking at Mars to reach orbit is a risk. Braking there with a (bigger) solar engine instead, followed by a chemical one, would need 280t on Leo. The departure chemical stage can weigh 160t for 2600m/s if the vessel and departure solar stage total 120t, and be launched separately to Leo. Unfolding the concentrators gets easier. Presetting the descent-ascent module took fourty-four concentrators, the return vessel as well. Joining them on Martian orbit would improve the acceleration of the return vessel. The twenty solar engines of the crew's leg to Mars could be separated earlier before Mars and have a bit of hydrogen more to brake autonomously. One more rendezvous with the return vessel, limited benefit. Keep the glider vessel for the return leg! This saves 20t from 62t preset in Martian orbit, and since the leg to Mars needs less launch capacity, the vessel can grow. Marc Schaefer, aka Enthalpy Nice of you!
-
Would you tell more? Whether you want to analyze traces of isoprene in air, remove isoprene vapours from the breathable atmosphere in a working area, or even produce isoprene from tree emissions in a plantation, the method will differ! Especially whether you want to obtain the isoprene (condensation, solvent, adsorption) or just remove it destructively (ozone, UV, catalyst, filter?).