-
Posts
3887 -
Joined
-
Last visited
-
Days Won
1
Content Type
Profiles
Forums
Events
Everything posted by Enthalpy
-

Producing energy from the pressure at the sea bed
Enthalpy replied to LoneWolf's topic in Engineering
Anyway, the idea of removing water from a tank with external pressure is bad, because the tank must resist the pressure, and worse, external pressure, and this costs an awful lot to build. Much more clever - and I believe economically viable - are Prof. Seamus Garvey's underwater bags http://news.bbc.co.uk/2/hi/uk_news/england/nottinghamshire/7315059.stm http://www.energyharvestingjournal.com/articles/compressed-air-energy-storage-00003358.asp?sessionid=1 which are being prototyped. These have bags anchored at depth, which don't resist the pressure. They store air as a trapped bubble, pumped or turbined to store or recover energy. If you compare both: - For the save P*V, the evacuated tanks must resist the pressure, the bag not! Bags win, tanks lose. - You get a bit more energy from an expanded gas, because its V increases when you exploit its P - Proper heat exchangers can give the air the temperature of the Ocean's depth when you compress it, but of the Ocean's surface when you exploit the expansion. I tried to put some technical and economic figures on the bags (and pumps and pipes and exchangers). http://www.physforum.com/index.php?showtopic=21016&st=0 sorry for the mess I believe these belong to the very few sensible methods - together with flywheels http://www.scienceforums.net/topic/59338-flywheels-store-electricity-cheap-enough/ and just batteries (preferably of sodium instead of lithium if the Japanese university succeeds) plus of course pumping between two lakes (already done, needs lakes) and possibly the Belgian artificial ring island on shallow seabed, from which water is removed to store energy. "Self-powering" sounds like energy created from none, which doesn't exist. As opposed, electrolyse water to store electricity, consume the gases in a fuel cell to restore it, that works. It's just that both transformations are inefficient, like 60% each. Such figures are a no-no in the electricity business. Big water pumps and turbines achieve >>95% which is a more sensible figure. The flywheels must be the most efficient over few days. -
For a single user, you could cast a foam to the body shape. Is it done for race drivers? For many users, a water bed maybe? That will work at horizontal surfaces.
-

Can you carry more info through lasers than on radio waves?
Enthalpy replied to HRS's topic in Engineering
Many experiments are under way among space probes and satellites, exactly because designers hope to increase the data throughput using lasers. The main reason is that light can be focussed to a narrower beam than radio waves using an antenna, lens or mirror of limited diameter. An other reason is that much mandwidth is available, which helps (a bit) transmit more throughput from the same link power budget. All the rest is worse with light: emitted power, receiver's sensitivity, modulation capability. A narrower beam demands to point more accurately the transmitter and receiver - much so before improving over a radio link. This has been a hard nut up to now. One option is to transmit by light (say from a deep space probe) to a satellite on Earth orbit, then relay somehow to Earth: this eases weather requirements. I've suggested to pump the transmitting laser by sunlight directly, for efficient conversion: no electricity in between. Yag lasers absorb sunlight well with a second absorption center (is it terbium in addition to erbium?): a Japanses university does it on Earth with >20% light-to-light conversion. For all data transmissions by light, on Earth or not, especially unguided, my filter would attenuate noise: http://www.scienceforums.net/topic/74445-evanescent-wave-optical-filter/ -

Gas Generator Cycle for Rocket Engines - Variants
Enthalpy replied to Enthalpy's topic in Engineering
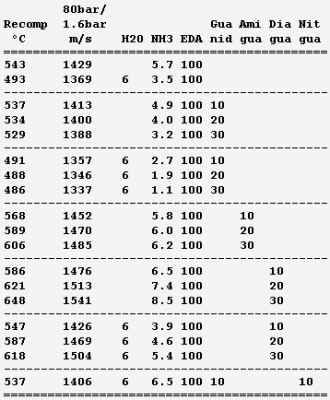
In gas generators that recompose a liquid, added ethylenediamine and ammonia have drawbacks (toxic vapours, viscosity, melting point) hard to guess on the paper. A more seducing option is to combine the dinitramide with a cation heavier than ammonium. Methyl-, trimethyl- and dimethyl-ammonium dinitramide improve the performance and keep a reasonable pH. Dissolved in water for 700°C or 1100°C, they expand to 1550 and 1850m/s, while from 700°C, added ethanol must thin them, and from 1100°C, gains 100m/s if the mix isn't too thick nor flammable. Guanidinium isn't so good, but di- or tri-aminoguanidinium nitrate replacing 10% dimethylammonium dinitramide avoids alcohols and keeps 1900m/s from 1100°C. Tri- or di-aminoguanidinium dinitramide must be more soluble and efficient, if practical. These aqueous gas generators are second to toxic hydrazine but outperform the explosive hydrogen peroxide by far. Marc Schaefer, aka Enthalpy -
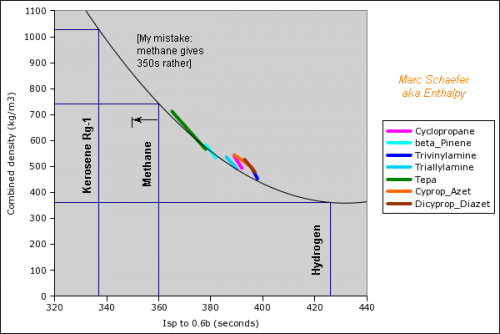
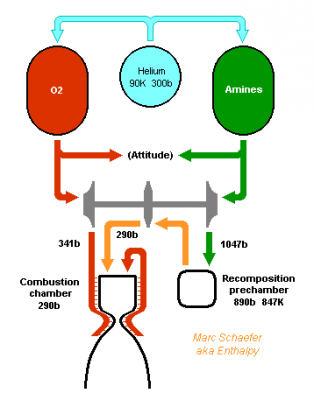
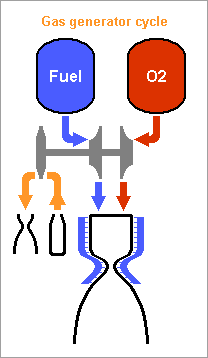
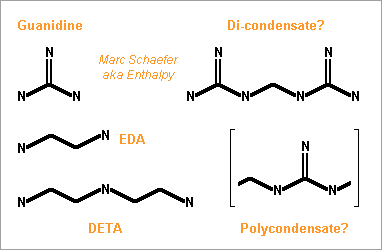
Hello you all! Many rocket engines pump liquid propellants to their combustion chamber, and varied cycles are used to power the pumps, the best known being: Gas generator http://en.wikipedia.org/wiki/Gas-generator_cycle_(rocket) Staged combustion http://en.wikipedia.org/wiki/Staged_combustion_cycle_(rocket) Expander http://en.wikipedia.org/wiki/Expander_cycle_(rocket) but here I'd like to describe uncommon cycles, which may be new and of my invention. ===================================================================== The first sketched cycle realizes the cracking (hydrogenolysis) in a pre-chamber of a hydrocarbon with hydrogen, which produces methane, some excess hydrogen, and enough heat that the following turbine powers all pumps that achieve for instance 440b in the pre-chamber and some 200b in the combustion chamber. An amine, imine, nitrile can replace the hydrocarbon: Logically, fuel density and specific impulse are between methane-oxygen and hydrogen-oxygen, thus filling the gap between kerosene and hydrogen engines: The pre-chamber and turbine run with fuel-rich hot gas, far easier than the staged combustion of hydrocarbon fuels which was oxygen-rich to avoid soot. Marc Schaefer, aka Enthalpy ===================================================================== Now, this other cycle decomposes an endothermic oxidizer in a pre-chamber to obtain hot gas for the turbine. All the oxidizer flow decomposes and continues to the main chamber. It shall be simple and reliable: No mix is required at the pre-chamber, which runs at a safe fixed temperature (but in oxidizing gas, true) Pump and turbine speed is moderate If you have some limited pressure in the tanks, opening two valves starts the engine, and the attitude control can be pressure-fed The oxidizing gas lights the fuel by its mere temperature The moderate decomposition temperature permits a sort of glow-plug igniter This cycle burns storable propellants and is more efficient than tetroxide and toxic hydrazine, like 350s with a good expansion, and its tanks are lighter. One oxidizer is Mon-33, or 33% NO dissolved in 67% N2O4. It freezes at -107°C, so if paired for instance with 2,4,6-trimethyl-tridecane (freezes at -102°C), they stay indefinitely on Mars or an asteroid or the Moon just in white tanks. Less NO lowers the vapour pressure at terrestrial temperatures, lowers the pre-chamber temperature, and loses little performance. Hydrogen peroxide H2O2 is used that way by the RD-161P engine: http://esamultimedia.esa.int/docs/EMO/LPE.pdf but to this option, I prefer a liquid oxygen staged combustion, because peroxide isn't really storable and is dangerous. http://www.gkllc.com/lit/gk-authored/AIAA-2004-4146_Field_Handling_of_hydrogen_peroxide.pdf Marc Schaefer, aka Enthalpy ===================================================================== And that other cycle recomposes in a pre-chamber a mix of amines to produce methane, nitrogen, little hydrogen, and heat: Few amine mixes don't soot. I consider Ethylenediamine dissolving 358:1000 of Guanidine. The pre-chamber, turbine and pumps work then at comfortable temperature and speed, and the hot gas is fuel-rich. Ethylenediamine NCCN or C2H8N2 is liquid between +9°C and +116°C, nearly safe, and its recomposition produces a temperature over the decomposition and the atmospheric autoignition, helping to recompose in the pre-chamber. But it needs some hydrogen supplement. Guanidine N=C(N)N or CH5N3 melts at +50°C and decomposes at +150°C. If 358:1000 dissolve in Ethylenediamine, sooting is overcome, and they hopefully lower the freezing point. Note: I've taken published -56kJ/mol heat of formation but doubt it; +56kJ/mol would soot. Methylamine NC or CH5N boils at -6°C and its recomposition is reputedly unstable, but 240:1000 in Ethylenediamine overcome sooting and should lower the freezing point. Guanidine and Methylamine dissolved both in Ethylenediamine may combine the best freezing point and vapour pressure. The sketch suggests an optional pressure-fed attitude control, but since only oxygen gives good performance here and is more difficult to store, this cycle would rather fit some launcher's lower stages. Attitude control uses to gimbal engines there, and booster pumps accepting a low input pressure save tank mass. This cycle is as good as oxygen-kerosene in a staged combustion but far simpler. Marc Schaefer, aka Enthalpy ===================================================================== For the cycle that recomposes an amine in a pre-chamber, Methylamine (MA) is corrosive and boils at -6°C, yuk; to avoid soot, Ethylenediamine (EDA) would need 100:23 of dissolved Methylamine (yuk) or 100:36 Guanidine, but the solubility and the resulting viscosity are doubtful. Alternately, Ethylenediamine could dissolve just 100:3.2 of Ammonia to suppress sooting. I've taken the heat of formation of liquid ammonia for want of the solute. Ammonia is nasty, but 3% should produce little vapour pressure - less than 100:23 Methylamine. Once Ethylenediamine contains the Ammonia bit, one can add Guanidine, Aminoguanidine or Diaminoguanidine. http://en.wikipedia.org/wiki/Aminoguanidine (beware 1720kg/m3 matches software divination) Aminoguanidine=Pimagedine was tested at 300mg/day as a drug, so it isn't very toxic. More aminated Guanidines bring heat (smoother decomposition; molybdenum turbine if needed) and performance but can't replace Ammonia - at the non-soot minimum amount in the table. In the table, added Guanidines only keep the exhaust speed of Ethylenediamine + Ammonia at best, because this comparison is at identical pressures: 700bar in the recomposition pre-chamber, 300bar for combustion in Oxygen, 0.5bar at the exhaust. But: The expansion speed from the pre-chamber improves. Power from 820m/s vs 769m/s adds 14% pressure. The fuel is denser (EDA 900kg/m3, GUA allegedly 1550kg/m3) and it needs less oxygen - both increase the pressures. 30% more pressure brings much more than the apparent 1s loss. Denser propellants make also lighter tanks. This idea fits also the fuel-rich staged combustion (if any useful) and the full-flow staged combustion, in an other thread. Marc Schaefer, aka Enthalpy
-

Gas Generator Cycle for Rocket Engines - Variants
Enthalpy replied to Enthalpy's topic in Engineering
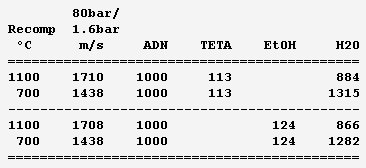
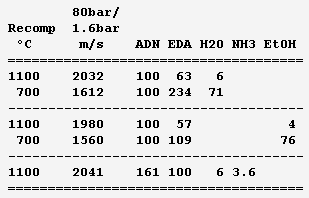
Ammonium dinitramide "ADN" (NH4)+[N(NO2)2]- is a fashionable solid oxidizer: H4O4N4, 1810kg/m3, Hf298=-148kJ/mol but dissolving adds 36kJ/mol, mp+93°C. Solubility in water is 3.57kg/kg. It's a seducing replacement to ammonium perchlorate in solid grains and a discussable replacement for hydrazine and tetroxide - I'd switch to electric propulsion or to oxygen. It looks good here as an auxiliary gas generator to rotate a turbopump. It brings H, O, N so its recomposition makes sootless gas, at 1800°C so the turbine demands dilution - hence as a liquid propellant. ---------- Here stoechio amounts of fuels are added in water. All fuels (ammonia, diaminoguanidine, EDA) gave me the same performance since the gas is mainly vapour: safety and practical consideration (melting point) can decide. These mixes make hotter gas than those based on EDA for the same performance, but they would achieve temperatures fitting (hypothetical) molybdenum turbines, and be then more efficient. They have a lower vapour pressure and are also denser. ---------- Now a fuel-richer mix produces methane and some hydrogen in addition to vapour. The expansion speed improves, but the propellant is volatile and possibly viscous, provided so much ADN dissolves. Water and ammonia prevent soot here. The previous mixes are safer. ---------- The previous injector scheme may keep useful. Other solid oxidizers are allegedly better http://www.chemicalforums.com/index.php?topic=74828.0 but I got solid data for ammonium dinitramide. Marc Schaefer, aka Enthalpy -
When the brake looks like a bearing, it can also have rigid rolling elements and viscoelastic races. Or maybe viscoelastic rolling elements and races. Marc Schaefer, aka Enthalpy
-
[i add labels] (A) Nuclear electricity is presently more expensive than wind electricity, which in turn is much more expensive than gas and coal. As well, many uranium-consuming countries don't mine it locally. So nuclear electricity is an economic and a military weakness. (B) Wars are of varied nature. Imagine when European and American countries attacked Libya to overthrow Ghaddafi: he wouldn't have cared about neighbour countries, didn't consider invading Europe - but had he had the proper weapons, he definitely would have threatened to burst a few reactors in Europe and would have won the action. This is an example where conventional weapons would deter a country that has nuclear reactors. [C] So true, how much I agree with you! Legal limits are always raised by the government when a reactor leaks. When drought didn't permit to cool France's reactors properly, the gov just increased the acceptable river temperature rise by the reactors. After Fukushima, the UE increased the acceptable radioactivity in food. Liquidators at Fukushima must accept doses that were forbidden prior to the disaster.And so on. Fact is that a gov has no choice in its reaction to a nuclear incident. Giving up electricity is no good option. Leaving the country neither. Stopping eating, of course not. So the gov raises the limits. It doesn't make the pollution safer. It just shows that legal limits protect nobody.
-
[Enthalpy: radioactivity spread to users together with heat] And this accident would happen. Of course. The basic notion about safety is that accidents do happen. Prior to Fukushima, dozens of studies proved that losing all cooling methods would have been ludicrously improbable, with a coma and many many zeros as a percent probability. But it happened a all six reactors of dai-ichi, all reactors of dai-ni, and several more plants. Te other reactors could be cooled because an ultra-paranoid commission had thought that a tsunami could stop all cooling, and that the Diesel generators would be transferred within the building, which had not been done timely at the four exploded reactors. The interesting question is not what precautions have been taken, nor how improbable someone imagines or claims the accident to be, but what its consequences are, precisely because accidents do happen. Pipes from a hyper-deadly site like a nuclear reactor and the population are just no-no. The number of alleged barriers is irrelevant there.
-

Gas Generator Cycle for Rocket Engines - Variants
Enthalpy replied to Enthalpy's topic in Engineering
True, it's not directly a turbine speed. It can be a gas speed under some circumstances, not uncommon, where the turbine is of Laval type. It does tell, through m*V2/2, how much work is extractible from this secondary flow - before the turbine introduces its losses. It also tells very much how the turbine(s) must look like. For instance, if one expands hydrogen (heated to 600°C by a little bit of oxygen) from 100bar to 5bar, it acquires 2792m/s, while a flat Inconel disk explodes around 460m/s, and an oxygen centrifugal pump needs 120m/s - by seeing such figures, a turbopump designer grasps that the turbine will be inefficient even with several stages. -
I've checked the wiring cables on aeroplanes, and apparently they are still made often of copper, aluminium being the exception, for reasons I ignore and can't even guess. Very bizarre to me, since most high-power terrestrial cables are of aluminium. Houses had worries with alu cabling 50 years ago, but that's over, and plane manufacturer would easily afford to develop their own connectors for aluminium if it were any necessary. I'd really prefer to wire aeroplanes with aluminium (Elec. Eng. was my first profession), and with the gained mass, have separate data networks on the 777 and Dreamliner to fly the plane and to carry passengers' data. I'm not presuming MH370 was taken over by a malicious software: while this would fit what the public knows of this incident, there is no element to decide for or against it. Just to tell that a common network is foolish to my opinion, and that malicious software will take control some day if not at MH370.
-

Gas Generator Cycle for Rocket Engines - Variants
Enthalpy replied to Enthalpy's topic in Engineering
Oops, you're right. ---------- Combustions use to be too hot for turbines. Typically over 3000K with liquid oxygen, while nickel alloys work at 700°C. A main combustion chamber can have its walls cooled by a propellant flown through a jacket, but at a rocket turbine, this would be too complicated (it's done at aeroplane engines to gain ~100K operating temperature). This is a big difficulty at rocket turbines. One answer is to detune the propellant ratio there. Easy with hydrogen, difficult with hydrocarbons: more fuel makes much soot before the temperature is bearable, more oxygen tends to burn the metals, the joints, everything. What's worse, a flame is difficult to stabilize at such a low temperature - one answer is to burn hot and dilute later - and the mixture and flame can be heterogenous, with some spots dangerously hotter, or with hotter periods over time. So a single propellant - which avoids the uncertainties of mixing - the produces naturally a mild temperature would be welcome, even more so if it doesn't soot. This is the case with hydrogen peroxide (used from the V2 to the Soyuz) and with hydrazine, but both are dangerous. The amines I suggest are caustic, but at least no brutal carcinogens, and hopefully won't detonate. ---------- The speed I indicate is for unhindered gas expansion, in this case from 80bar to 1.6bar because I found such pressures meaningful. The speed essentially indicates how much mechanical power is available in a mass unit of the produced gas, hence how much of this additional flux is required to rotate the pumps for the main propellants. In some turbine designs, the gas is completely expanded before the turbine rotor, where only the direction change provides a force, a torque and a power. There, this speed can apply directly. In many designs, the expansion occurs in part in the rotor, or is done over several stages. Then the speed indication tells a designer if the turbine speed will match easily what the turbine and pump materials survive (that's a big worry with hydrogen) and if it will fit easily what the pumped propellants need. If not, multiple stages may bridge the gap, more or less well. -
Please see my answer on the other forum.
-

How many planets in how little space?
Enthalpy replied to Moontanman's topic in Astronomy and Cosmology
Several Earth-sized planets within Mars' orbit is possible: Venus and Earth proove it. About software, be very cautious: instability uses to result from the software rather than from celestial mechanics. -

Material disintegration by cold?
Enthalpy replied to MirceaKitsune's topic in Modern and Theoretical Physics
Electrons have no speed around the nuclei. They use to be immobile, but have a volume because they're waves, and this volume can encompass several atoms, in which case the atoms are bound. "Spin" is something else. Not related to the temperature, neither. Heat is the movement of the atoms, which electrons can limit if the temperature isn't too hot. Electrons don't participate to it in most solids at usual temperatures. As the bonding force doesn't result from electron movement, and heat isn't essentially electron movement, extreme cold doesn't disintegrate solids this way. Other processes exist, for instance if one region of a solid shrinks at cold while an other region is still warm, the difference of contraction can break the solid. -

Deterministic interpretations of quantum mechanics?
Enthalpy replied to Fanghur's topic in Quantum Theory
Deterministic interpretations are abandoned. -
I had a strong background on waves (electrical engineering and acoustics) before starting QM, it helped me a lot. Learn as much as you can there, including optics, acoustics, if possible radiowaves. In maths, linear algebra and Fourier transforms - to begin with, as there is no limit... Hamiltonian: I saw it first with QM, and I too wish I had seen it at work in classical mechanics before. Electromagnetism is important independently of QM, and interesting, so learn it anyway. It's difficult (more precisely, antennas are difficult, just like music intruments are the difficult part of acoustics) but less abstract than QM. I strongly suggest that you learn through experiments kits in parallel with books or before. They are often very well made and give a different comprehension of science, one that is more useable than textbooks. They exist for electromagnetism and for optics at least. About QM: many, many people tried to explain it using comparisons or images, but these ("surfer and wave at the same time", or the "wave or particle duality" dialectics...) only add complexity. Just go straight to the mathematical description.
-
Yes it does, experimentally. Both the frequency of the light and the interval between the pulses.
-
Before the loudspeakers, you have amplifiers, whose input at the signal connector has a rather high impedance. This makes them sensitive to electric fields rather than magnetic fields. he loudspeakers alone give zero noise under these conditions. Any conductor pick up electric noise created by the environment, the 50Hz/60Hz mains being one common source, radio and TV emissions an other one. Against that, the signal cable to the amplifier is (too poorly) shielded, but the unplugged connector is not, so it catches ambient fields. A human body catches fields better than the connector does because it's bigger. With one hand near a lamp you catch the field, with the other you inject it (or the voltage, and so on) in the amplifier. Electric noise pickup is a basic annoyance of any analogue electronic design, and digital as well. Combating it is non-trivial, both from electromagnetism and experimental knowledge; this know-how should have been part of the formation of every electronics designer, but it's not, alas.
-
One absolute limit to sound wavelength is the distance between atoms. It limits sound in air to <<1MHz and in solids to a few GHz - such frequencies are indeed used in nondestructive testing. This is very, very far from visible light (500THz) and gamma rays (again 100,000 times more). Many loss mechanisms can, and use to, limit the frequency much earlier than the interatomic distance does.
-
Visible light is typically absorbed by an electron that spreads over many atoms - the chemical bonds are called "conjugated dienes" as an approximation.This electron can have different shapes; it uses to take the most stable one but light can deform it to a less stable shape. In fluorescence, the electron soon regains its most stable shape and emits a photon (light) in the transition process, with a colour defined by the energy difference between both electron shapes.
-
Sublimation depends on the material. Cadmium and zinc for instance are not used on a spacecraft because they sublimate too quickly. That's a serious worry because both are useful coatings on steel, and are commonly used on terrestrial parts, which therefore can't serve in space. Besides sublimation, some polymers (PVC especially) lose water in vacuum and shrink then, up to 3% in each dimension, so that parts wouldn't function any more. PVC is excluded from spacecraft - one more common material found in many terrestrial components, like electrical wires. Erosion by residual atmosphere is a real concern on low-Earth orbit, like 200km to 400km altitude. It won't punch a hole through a metal wall, but it does change the colour of the outer surfaces, which are essential to define the spacecraft's temperature. Other materials fail quickly in space because of UV light or ionizing radiation, rather than vacuum.
-

Gas Generator Cycle for Rocket Engines - Variants
Enthalpy replied to Enthalpy's topic in Engineering
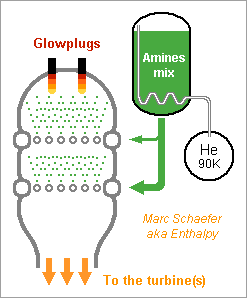
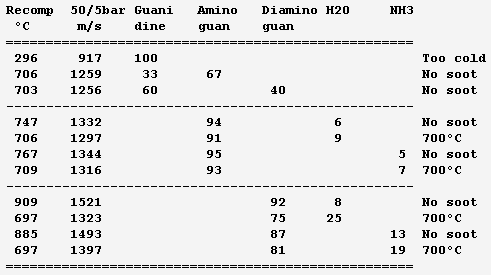
For a gas generator that recomposes a single liquid, here are mixture attempts based on ethylenediamine (EDA). Solubilities and resulting viscosities are unknown. Solutes use to improve liquid ranges. Ammonia is dissolved, the minimum that avoids soot at 80bar; unpleasant enough, and more would reduce the performance. The optional 6% water bring pure ethylenediamine's melting point from +11°C to a -1°C eutectic; more would worsen. Dissolving loses 11cal/g heat of formation but saves ammonia and should raise the flash point from +43°C and reduce the vapour pressure, includig from ammonia. The effect on viscosity is unclear. http://www.dow.com/amines/pdfs/108-01347.pdf Guanidine loses some expansion speed put increases the density, so that more pressure can compensate, and saves ammonia. Aminoguanidine gains expansion speed and recomposition temperature, but needs more ammonia. Diaminoguanidine even more so. Less diaminoguanidine outperforms more aminoguanidine. Nitroguanidine would need much ammonia, more so than diaminoguanidine for the same performance. The expansion speed is from 80bar to 1.6bar as throttled from 120bar, a good value for graphite-fibre tanks and helium pressure feed that helps start and restart. Nickel alloys withstand the recomposition temperature, which exceeds EDA's autoignition in air (385°C) but is little for a quick reaction; injection against the gas stream may help - expect hotter regions then. Staged injection favours a stable recomposition. The helium tank is smaller if coupling it thermally with the oxygen tank. The glowplugs can be from a Diesel engine if big enough. Marc Schaefer, aka Enthalpy -

Gas Generator Cycle for Rocket Engines - Variants
Enthalpy replied to Enthalpy's topic in Engineering
Erratum. Guanidine isn't knowingly volatile, my mistake. Meanwhile I doubt Aminoguanidine's 1720kg/m3 at Wiki because it matches software divination. Aminoguanidine must be dense, but is there a measure? -
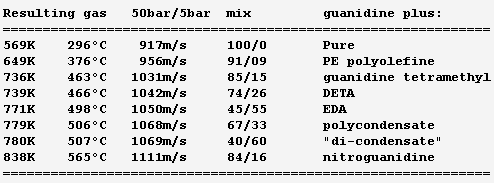
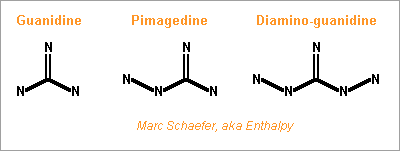
Hello everybody! The gas generator cycle is widely used on rocket engines, but some variations are still possible and desireable. For instance, the Falcon launcher burns the derived flux with much kerosene and little oxygen to limit the turbine's temperature, but the resulting soot complicates the reuse of the engine. One alternative, similar to Ariane's Viking, would add water to a tuned mix of RP-1 and oxygen, injected in two steps. 107:10:34 results in 873K=600°C; expansion from 50bar to 5bar gives 1133m/s. Good solution with one pump more. If accepting additional propellants, (yuk!) hydrazine or MMH, hydrated for 600°C, give 2004m/s and 1696m/s - not fully useable by a turbine. Methylamine (yuk as a volatile amine, not carcinogenic like hydrazines) gives 400°C and 1552m/s if its decomposition is even. Hydrogen peroxide, 84% for 600°C, gives 1099m/s but can detonate. ---------- I consider here a solid gas generator. It starts more easily, and mobile inlet vanes at the turbine can control the generator's pressure hence "burning" speed through the pressure exponent. Blowing upside makes debris less critical. One could add gelled water to smokeless powder. For instance 123:70:30 water, nitrocellulose and nitroglycerine gives 873K=600°C and 1125m/s. Expect some particles from the gel in the gas. One solid that makes moderate temperatures and no soot is guanidine: pure, it gives 917m/s and 569K=296°C - supposedly too little for a stable "flame". Guanidine may also be brittle. It's a deliquescent and caustic base that melts at +50°C. I take -56kJ/mol from a single source. Here are some additives to make a hotter and more stable "flame" and to make the block tougher - hopefully... Only experiment can tell. The mix ratio here is the soot limit. Guanidine tetramethyl, EDA (ethylene diamine) and DETA (diethylene triamine) are liquids. The hope is that they can dissolve or impregnate guanidine, evaporate partially if needed, and leave a shock-proof gummy thing that "burns" better. PE (polyolefine) would just cover guanidine grains, to bind them together and as a moisture barrier. Maybe the polyolefine is first dissolved in a small alkane that evaporates. Other polymer candidates, like varnishes, must bring little oxygen, and resist the strong base. If guanidine reacts with formaldehyde (or with ethylene glycol) like melamine does, and the water can be removed, then some "di-condensate" (isomers) shall raise guanidine's melting point and the "flame" temperature. In contrast, the cross-linked polycondensate macromolecule could be formed at the surface of guanidine grains, as a moisture barrier, flame improver, and binder or binder primer. Nitroguanidine brings heat and performance; combine with the previous ones if possible. ---------- Pumping 1t of RP-1 and oxygen to 100bar, with 74% 79% 88% efficient pumps, turbines and injectors, needs 37kg of gas at 1000m/s, which still blows at 750m/s after expanding from 5bar to 1bar. A solid gas generator adds only an envelope; at 700MPa for 50bar and 8200kg/m3, the envelope weighs some 4kg. Marc Schaefer, aka Enthalpy =========================================================================== Despite its N-N bond, Aminoguanidine (=Pimagedine) isn't toxic like Hydrazine, Mmh and Udmh are: test persons ingested 300mg/day when it was developed as a drug. http://en.wikipedia.org/wiki/Aminoguanidine 1720kg/m3 (wow!) and bp=+261°C, mp unknown. All are corrosive, caustic and deliquescent. Guanidine is worryingly volatile, but Amino and Diamino-guanidine little so. They bring more hydrogen for the same carbon, and much heat of formation, which I estimate to +60 and +177kJ/mol for the solids at 298K. Their hotter recomposition would make some soot instead of methane, worse at moderate pressure, but blends can avoid it and achieve an excellent gas speed. The blends with Guanidine are still volatile: consider coating them as previously suggested. More seducingly, tiny additions of water or ammonia suffice to avoid soot and, given their affinity for the strong polyamine, must build a solid blend. In the list, 700°C is the fuzzy limit of nickel alloys, but the molybdenum TZM alloy (which could improve the turbine of any pumping cycle) could reach 1100°C and obtain 30% more power, saving gas generator mass or improving the main chamber's pressure. http://www.plansee.com/en/Materials-Molybdenum-402.htm Water could be gelled, but I hope a better process can produce the propellant block. A carrier gas would bring vapour or ammonia between the grains of Amino- or Diamino-guanidine where they would settle and soften the surface; the block would be pressed, possibly in the final cartridge; water or ammonia would later diffuse to the depth of the grains to achieve a solid, while a light carrier gas like hydrogen or helium would exodiffuse. The cartridge can then be sealed for storage and be the pressure vessel at the rocket. This all depends on how the aminoguanidines recompose. Additives can help as usual, and fusible fibres can toughen the block. Aminoguanidines may serve in liquid blends as well, but more usefully in a staged combustion cycle, so I'll put a word there. Marc Schaefer, aka Enthalpy