Erina
Senior Members-
Posts
171 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by Erina
-
I have a wire mesh waste paper basket and my eyes confuse when moving my position while the basket is still as the two mesh layers overlap to create confusing patters as my eyes attempt to focus, which is most noticeable the farther away observed. It's nothing new, but what is this phenomena known as ?
-
How well is the human body's process understood if imbibing L-Tryptophan (in synthetic supplement form) that the liver will process only what the body requires to convert to 5-Hydroxytryptophan for use as serotonin? If skipping this step and directly introducing 5-Hydroxytryptophan into the body, it surely has no chance to regulate how much it needs?
-

Zinc occurs naturally in the human body ?
Erina replied to Erina's topic in Biochemistry and Molecular Biology
I understand that Zinc can be obtained from foods, but does it occur naturally in the body ? -

Zinc occurs naturally in the human body ?
Erina posted a topic in Biochemistry and Molecular Biology
I read that the human body cannot produce minerals, such as Magnesium, but the body does require them and so minerals must be absorbed topically via the sun, or imbibed/ingested through food and drink. However, Zinc is a mineral, but is found in the human body in the eyes and bones, only at trace levels of a single digit grams net weight apparently, but then how does it arrive there, does the body naturally replenish it ? -

Mould in silicone turning from white to brown after being bleached
Erina replied to Erina's topic in Inorganic Chemistry
I can confirm now that after six months, under the same wet kitchen use conditions, there has been no re-growth from the mould. I also tried this on white bathroom caulk around a stand up shower and it worked exactly the same, leaving the caulk looking like the day it was first laid. This is a solid technique and very easy the achieve. -

Mould in silicone turning from white to brown after being bleached
Erina replied to Erina's topic in Inorganic Chemistry
Surprisingly, after eight days, the silicone now smells sweet?! -

Mould in silicone turning from white to brown after being bleached
Erina replied to Erina's topic in Inorganic Chemistry
Interesting. I've only just done the experiment, so I'll have to see, but I'll be keeping an eye on it.. -

Mould in silicone turning from white to brown after being bleached
Erina replied to Erina's topic in Inorganic Chemistry
So why did it turn brown? -

Mould in silicone turning from white to brown after being bleached
Erina replied to Erina's topic in Inorganic Chemistry
I used just household bleach : "Domestos Extended Germ-Kill Citrus Fresh Toilet Bleach with CTAC 750ml" Ingredients : Cationic surfactant; Soap; Non-ionic surfactants; Disinfectant: Sodium hypochlorite 4.5g per 100g. < 5%: Chlorine based bleaching agent (Sodium Hypochlorite); Perfume. -

Mould in silicone turning from white to brown after being bleached
Erina replied to Erina's topic in Inorganic Chemistry
I assume so, I just wondered what the underlying chemical process was to turn it from white to brown? -

Mould in silicone turning from white to brown after being bleached
Erina replied to Erina's topic in Inorganic Chemistry
Well, it was only for 30mins in total, and I did have the window open (and I'm alive to tell the tale), but you're probably right. Any ideas on why it went brown please? -
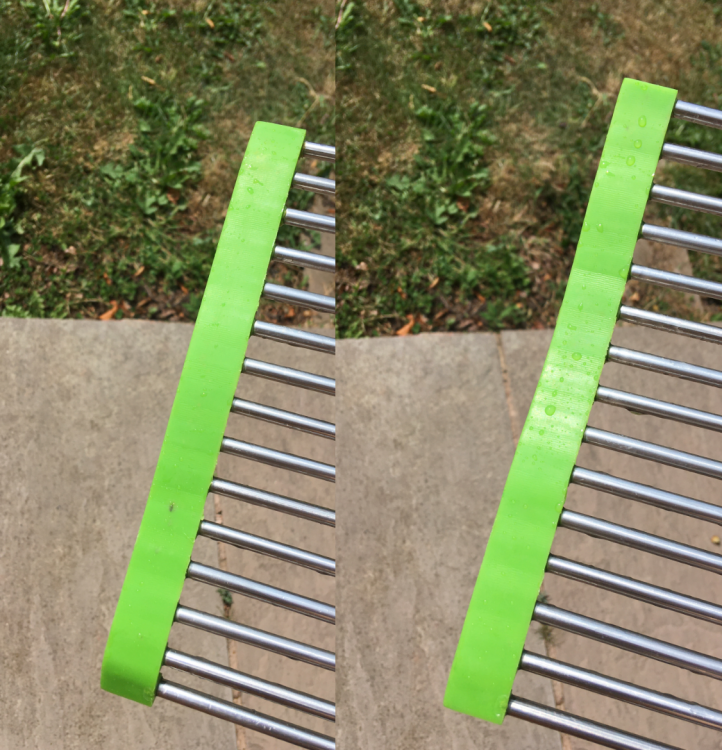
Recently I finally decided to rid my silicone/steel trivet of the mould that had been building up over the years through normal kitchen use after being left rolled up when not in use (a design feature). Submerging it in bleach for 24 hours the majority of the dense mould colonies succumbed to the bleach and disappeared: changing from black to white (or a lighter shade of the green silicone). After submerging them for 12 hours in a bicarbonate of soda bath to attempt to rid the silicone of the bleach odour, to no avail, I heated the silicone in an oven for 15mins at 120ºC (twice) in order to attempt to bring the oils (odour) to the surface of the silicone to be washed away. I didn't have too much luck with this and the baking process released a fairly acrid smell into my room so I didn't persist, but did have some success. However, now the white areas (where the mould once was) have turned brown, why is this?
-
Heating foodstuffs in a domestic electric oven is best achieved under conditions with an even air flow to spread the transfer of energy, typically using a venting system with a convection fan. However, could the same not be achieved solely with an internal fan to radiate the air inside? If so, then it could negate the need to puncture the internal membrane of the oven with fan and ventilation access, as a magnetic stirrer system could be employed to circulate the air. With an electric system there would be no need to worry about ventilation of gases as well. One issue could be the temperatures themselves, as I understand that magnets degrade under heated conditions, but perhaps there could be a material to better protect it from the heat?
-
Interesting read, I don't have access to the PDF however, but it seems like a lot of the thinking here coincides with what I was looking to achieve, although I have more flexibility as I don't plant to have the same size restrictions. I never thought about the width of the fibre optic diameter, that would probably cut down on the complexity, and looking into the matter of the shape they don't have to be circular either, I could use square cables and gain the otherwise missing negative space.
-
I would like to be able to passively project a humanly recognisable object over a dense array of fibre optic cables, over a distance of 100m, without the need for electricity. 1. To project more detail than a silhouette, light projection onto the object itself is required. Could diverting some of the cables toward a natural daylight source be used to sufficiently illuminate the object from a distance of 5cm? 2. If so, what percentage of the cables would be needed to illuminate an object 5cm away e.g. every other cable? 3. I assume that the array of cables is capable of transmitting the image distortion free if curved with the minimum bend radius of an fibre optic cable?
-
Kohler the company produce a simplistic perpendicular designed outlet, flush from the wall, which produces a crystal clear jet of water for use in the domestic bathroom environment: https://www.faucet.com/kohler-k-923-polished-chrome-modern-wall-or-ceiling-mount-bath-filler-with-95-inch-2-4-cm-orifice-from/f231619 I would like to know how much control could be had from a static system regarding where the flow would finish? I understand that there are very strict principles to be observed in order to achieve the Laminar effect and it doesn't seem like there would be much room for manoeuvre with this design i.e. only on and off? What if the water were a widened sheet design, such as a uniform waterfall, could there be more control over the rate of flow and direction, whist still maintaining the clear effect? I am interested in the single jet stream for use to wash my hands in a small bathroom basin, but utilise the sheet of for washing up cutlery and crookery in a larger basin.
-
Usually drinking water fountains are pathetic things and quite unappealing. Assuming that the fountain could be guaranteed to be clean (members only) people wouldn't be so afraid to use one. However, to make it more appealing, rather than a little winkle of a squirt, I think that the visual appeal of crystal clear Laminar flowing water would be a big step in disarming people's fears. However, the entire flow must be in a single direction to achieve this and I should imagine that such a sensitive area as the mouth wouldn't appreciate the full force of the water hitting it directly. However, if more spacial in design, such as with a spherical / mushroom shape, then the force should be greatly reduced, but the effect still appealing. My idea is to have a drinking fountain that splays out equally so that multiple users could drink water at the same time from a beautiful display that shouldn't hurt. First of all, I assume that these pumping machines can push drinking quality water? Also, I would like to better understand how the water reacts with light: I assume that while the sheet could be projected on to (as with the time in the Osaka City Station, Japan waterfall display) it would also be transparent and light would pass through? Would it behave as fibre optics do and only display the light at the termination point as gravity pulled it to the surface i.e. creating a highlighted perimeter circumference, which would require the light to bend with the water, or would the entire sheet glow? The design in the video below is not what I had in mind, I am simply looking for a single self contained stem poking up to produce the drinking water on command:
-
Many thanks for your help ! Assuming if the magnets were woven into an upper body jacket (to better distribute the forces on the human body) could there be another chute with magnets arranged to zip the jacket back up to the top for the next person without power? Similar to how a battery moves around the inside of a coil, but without a current.
-
Although it would be a leap of faith, how reliable would it be for a human to strap on an easy to wear magnetic jacket/belt and jump down a strait tube of which the temporary Eddie current would slow the person down according to Lenz's Law so they would reach the end of the tube at ground level reliably and without injury? Sort of life a Fireman's Pole, only without the pole.
-
In contact with a thermo-chromatic supplier I get the impression that compound can be fine tuned to different temperatures, although there is a little overlap, it could be tailored to reliably change around ~2ºC, however there is no market for such incremental steps as I would need. This too sounds like another system of which I would need to tinker with..
-
I see. Well, was my hands aside, I think that the Bicarbonate of Soda can do that in spades, so I'll stick with the bicarb. But good to know.
-
Studiot : I am not sure what the soap nuts would be useful for in the cleaning process, the only reason I tried "soap" before was because I wanted to see if it would bind the perfume to the clothing once dry. It did not work as my records show, and that was with a lavender infusion, which is quite strong to human noses. I have looked into making soap, your suggestion is the "hot" method, and I needed to have a facemark fitted for that, but there ins't a place near by I can achieve that, nor one that carried 3M products (they like to foist other brands on to customers as it makes them more money), I would therefore have had to buy all sizes to see which fit me best and it was just all to expensive. There is a "cold" method available, but I'm just not making soaps right now, and my experiment showed that the scent didn't linger as I hoped anyway. Fortunately I don't have stains on my clothes, anything too troublesome and I would make a paste from the bicarb, rub it on and leave it to work, repeating the process with an vinegar (acid) wash. If the stain needed more attention, then I would buy a commercial product - this never happens to me though, because I don't care about stains, stains are cool on mans clothing! From what I read, "soap" is used as topological lubricant to aid in the process of cleaning oneself, it doesn't actually leave anything "clean" (i.e. dirt removed), save for a vehicle to more the dirt somewhere else, but water does that anyway. The benefit of the two cycle system that I employ is that the acid wash (white vinegar : 5% acetic acid) will kill the bacteria build up, and the alkaline wash will bind to it and the water wash it all away, resulting in a neutral finish. That dirt removed is what I am looking for, and the perfume can be of my choosing as an afterthought, if I can find a way to make natural non-synthetics bind with clothing.. Strange: I buy in 5kg (~11Ib) bulk bags, portion it out and (used to) cook around 1kg a time, there is no high street commercial outlet that will give me a better deal than Amazon. So it's cheaper this way. But I'll stop cooking the batch now because of the crystallisation (I can't believe I never checked, perhaps it's the water outside of London?) and will just use it strait. • My main reason for coming here was to find out why the crystals form, and I think it's because the anhydrous powder compound drinks the water and thus solidifies, is that right?
-
As my present it a factory issued product and I have no contact with the manufacturer so I cannot be sure of the water to ethanol ratio, and as I cannot get access to it then it matter even less. I was just hoping that there was a standard table with this already measured? It will be a process of trial if and when I make my own. I would like to know what the liquid is inside the vials, any idea? I thought about pigmenting my own with a thermo-chromatic solution to better emphasise the heat exchange effect, so that the colour transitions to white, and vice-versa, after having fallen?
-
I think that the surrounding solution is a mix of water an ethanol as it conducts to the heat better than just water. I found the following to be suggested as to be less reactive alloy metals than most: Pt, Rh, Pd, Os, Ru, Ir, Ti, it would just be a matter of finding the cheapest. As all of the vials contain the same liquid density (I don't know what that solution is inside the vials?) then I could modify the range of reaction, but what are the weights for each temperature?
-
studiot: I see clearly from the diagram that Sodium Carbonate only becomes soluble as the surrounding heat rises, losing its hydrate in the process, this I can confirm. Looking further online it seems that the crystallisation process occurs as the dry powder is an anhydrous compound and in this case can absorb water, which then becomes the water of hydration and thus crystallises. So Sodium Carbonate crystallises because it's an anhydrous compound and it can't help it? ref: https://owlcation.com/stem/What-is-a-Hydrate-Chemistry StringJunky: Thanks for the suggestion. I actually did try that as I wanted to try to perfume my washed clothes. Using natural essential oil extract I found that the scent would evaporate along with the water during the drying process, the scent would need to be a man made synthetic to have any clinging power. I don't need to fatty activity in my cleaning process, if I need to remove dirt then I soak in a white vinegar and water bath to kill any bacteria, then wash away in Sodium Carbonate rinse to balance the pH. The agitation during the washing process removes built up "dirt", while the fatty deposits form the soap would only encourage stubborn bacterial growth (which is what Sodium Bicarbonate is good at removing), so best avoided.