Everything posted by studiot
-
Equilibrium between [math]SO_3 [/math](product) and [math]SO_2,O_2[/math] (reactants)
Dhamnekar Win's fraction with the quadratic is correct for (c) I have not checked the arithmetic of his solution.
-
The Volume Problem
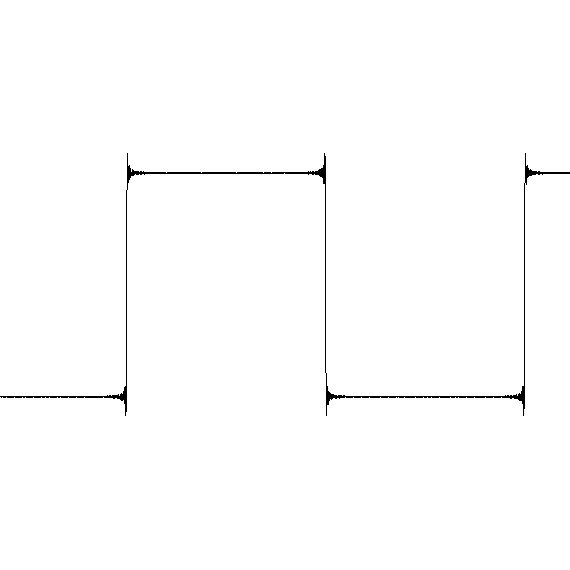
I don't see a problem here. The mathematics of the Ancient Greeks couldn't handle this question, and they developed many paradoxes involving their inadequate methods of analysis. Modern maths answers this as follows: Yes the spike exists, but it has zero duration in time. Incidentally you have a slight error in your maths. When you turn up the volume, you multiply by a positive number. But when you turn it down you multiply by a negative one. So the result is not x2 but x1 then by -1 = 1x(-1) = -1. OK so you have a spike of zero width. Or a pulse of zero width with a positive-going edge, followed by a negative-going edge. The point is that you only have one of these and 1 is a finite number. The Ancient Greeks' paradoxes all depend upon having an infinite count of these and they couldn't understand that an infinite count of these could add up to a finite total. We now call the pulse in question a 'Dirac Delta Pulse', which has some interesting and very important properties in modern Physics as well as pure Mathematics. Another example, that can be observed on an oscilloscope, is the fourier summing of sine waves to make a square waves. You can see what are known as 'Gibbs phenomenon' on the scope. As we approach more and more nearly vertical sides to the square wave, the leading and trailing edges extend further and further beyond the horizontal parts, culminating in spikes. The important point is that
-
how do you interpret multiplied units?
You and you teacher may have associated squaring with shapes but I will lay a bet that before your teacher mention s2, she said something like James has 5 apples. If Sally makes this up to five times as many, how many apples does James now have? You said as much yourself So how many square apples does James have ? Or is the 'unit' not square apples or (apples)2 ? Were you going to reply to my previous post ?
-
how do you interpret multiplied units?
Definitely not. Seconds squared although some may have got the idea from acceleration which is metres per second squared or (metres per second) per second. There is more in the Philosophy of Mathematics about this because we have to think of 'repeated operations' and also whether the output domain of the repeated operation is suitable for the next repeat. I am rather busy at the moment but will post more detail in due course.
-
how do you interpret multiplied units?
Welcome, I hope the various responses spread over a decade and a half helped but if you want to discuss this further please amplify and try to explain your exact difficulty as I for one couldn't catch it from your post. [aside] 'responses per decade' how's that for a unit ? [/aside] Please note two points about forum etiquette for this forum. We like members to make their point here, not tell others to go to the middle of somewhere else. Posting links is a great way to support something you have said here though. Also note the anti spam rule limit new members to 5 posts in their first 24 hours.
-
Is there any program that can convert letters to numbers and/or words into sums thereof?
Windows has voice recognition built in. Various programs use this, others use their own for arithmetic. https://www.dinf.ne.jp/doc/english/Us_Eu/conf/csun_99/session0224.html https://www.google.co.uk/search?hl=en-GB&gbv=2&q=windows+voice+recognition+do+arithmetic&oq=windows+voice+recognition+do+arithmetic&aqs=heirloom-srp..
-
How can information (Shannon) entropy decrease ?
+1 for the example to discuss. Not sure I see time in this, but this thought experiment on an ideal system introduces two interesting facts. Firstly in terms of classical Thermodynamics, the general equation for the entropy chance of such an expansion can be shown to be [math]\Delta S = {C_v}\ln \left( {\frac{{{T_2}}}{{{T_1}}}} \right) + nR\ln \left( {\frac{{{V_2}}}{{{V_1}}}} \right) = {C_v}\ln \left( {\frac{{{T_2}}}{{{T_1}}}} \right) + nR\ln \left( {\frac{{{P_1}}}{{{P_2}}}} \right)[/math] In this case since q = w = 0 no work is done and no heat is exchanged with the surroundings Therefore [math]\Delta U = 0[/math], and thus for an ideal gas T2 = T1 Yet there is a non zero entropy change, due to the volume change alone since the integral of q/T must be zero. Thius there is more to entropy itself than just the integral of q/T . Comparing this to a digital computer system let us say we have such an ideal computer and the analog of volume would be memory capacity. If we suddenly doubled the memory capacity, would there be a corresponding Shannon Entropy increase, since we have added nothing to the contents of that memory by the expansion ? And how does information fit into both these scenarios ?
-
How can information (Shannon) entropy decrease ?
also @joigus I have been looking round the net for information about Arieh Ben-naim and he seems to be a bit aside from the mainstream. I downloaded this pdf of one of his aticles where he seems to spend a lot of effort attacking the works of others, rather than developing his own stuff, especially when it contains some rather suspect statements. https://www.mdpi.com/1099-4300/21/12/1170 I would consider the book disappointing if that was all or the bulk of it's contents. Back to the subject of entropy. Ask yourself which group of people are most concerned with entropy ? Answer those who have compiled very extensive tables of values and use them in their every day work. Chemical Engineers, Mechanical Engineers and other Engineers directly concerned with Themodynamic processes. The forerunners of these people were those who originally introduced and defined entropy for very practical purposes, a hundred years before Shannon. One thing those steam engineers invented was called an indicator, which was a device to 'indicate' steam pressure. This led to the birth of the 'indicator diagram which is a plot showing the energies involved in a particular process. Originally these were P - V diagrams, the area under which indicates work. It was desired to introduce a variable that coupled with temperature to indicate energy and that is why entropy was conceived and introduced and what you will find on modern day's entropy tables. In modern times we have found quite a few pairs of variables that yield the dimensions of work or energy when multiplied together and also that it is useful to prepare 'indicator diagrams' for each of these.
-
How can information (Shannon) entropy decrease ?
Now that's a really good response. Thank you +1. It seems serious stuff from the contents list, quite at variance with the overdramatised brash headlines from Amazon. I find myself with much more interest in reading this book. Do you have any idea of the difference between this one and his others on the same subject ? Meanwhile have you heard of this book ? It has a similar theme and ambit. Here is a very interesting timeline found in Appendix IV Finally I don't know if you were here when I last stated the simple original reason for introducing the entropy function, which makes it so easy to explain. Have you heard of indicator diagrams ?
-
How can information (Shannon) entropy decrease ?
Any chance of a glimpse of the contents page at least and a review would be nice. I can't find these anywhere and I am unwilling to risk £20 to find out. The author seems to have written several books about entropy, but I can only find a couple of sentences quoted. These do seem to make sense to me however.
-
Maybe someone can help me solve this task, I really need help, please reply
tato has posted this on several mathematics forums, but has yet to receive a definitive answer, despite efforts by plenty of respected mathematicians. There is a geometric solution to a similar problem posted but although the diagram is the same different linking sides were involved as equal.
-
Politicians change Highway code...A poisoned chalice?
A pretty fair summary.+1
-
Prepare nutrient solution
Whilst your recipe list is (a bit) interesting you haven't said how much solution you require or what the form of your ingredients are. Considering you are not requiring analytical chemistry accuracy I think you could just scrape into the pharmaceutical balance category. Most of your ingredients require millimoles per litre which would cause no difficulty. The heavy metal sulphates have a MW of around 160 so you would be requiring about 0.1 - 0.2 millig/litre So if you could accept making up a large solution of these and pipetting or buretting some you could maybe get away with that. https://www.pharmaguideline.com/2014/09/calculation-for-weighing-range-of-balances.html If you don't know how to use a balance, you need technician assistance. Here is a standards pdf on preparing mixed solutions. https://www.training.nih.gov/assets/Lab_Math_II_Transcript_-_508.pdf
-
How can information (Shannon) entropy decrease ?
@Ghideon and @joigus Thank you both for a useful continuing scientific conversation. +1 apiece. There doesn't seem much else scientific going on here at the moment. I think Shannon entropy is specified and calculated on the basis of 'ideal' computers devoid of earthly defects, including the need for power sources. This is nothing new and continues the Ancient Greek tradition of abstracting perfect circles, squares etc as 'ideals'. We have carried this tradition on thoughout history in both Philosophy, Engineering and more recently Physics. In particular physical (ie thermodynamic) entropy and other thermodynamic properties are calculated on the basis of 'perfect' or ideal processes. Ghideon's comment about time applies if he doesn't already know this as the equations are almost all derived on the basis of infinitely slow (ideal) processes called reversible ones. thermodynamics doesn't care how long it takes to get there. One difference is that the thermodynamic statement "Ideal entropy cannot decrease" is defined for a cyclic process. It does not forbid entropic decrease within a cycle and this actually happens in some practical situations. Computing processes are not, in general 'reversible' in the same way.
-
Great Oxygenation Event: MIT Scientists’ New Hypothesis
In fact this work seems to be a development of the work of Berner (reference 45 in the paper) and Canfield (reference 1 and the paper)
-
Theory of Computation
What would be really useful would be a summary of what the course is actually about since 'computation' means different things to different sections of the maths community. That would be useful to others (like me) who might actually be thinking of looking at it. Thanks.
-
Difference in reaction between ferrous bisglycinate, ferrous gluconate, and ferrous fumarate
Ok so the fumarate and gluconate are acid ferrous (ironII) salts, whereas the bisglycinate is a chelate complex. I don't know how you prepared your solutions, but the salts will have water of crystallisation. For example the gluconate will probably be the dihydride. All this will affect the apparent molecular or equivalent weight in titration. Some interesting data about these here, but not necessarily your answers since we don't do your homework, just try to help. https://www.sabm.org/wp-content/uploads/2019/01/2A2-PhysiciansGuideOralIron.pdf
-
Great Oxygenation Event: MIT Scientists’ New Hypothesis
Our knowledge of oxygen processes in the atmousphere, land and oceans is, not surprisingly, far greater for the past half a billion years than for the previous nearly 4 billion years. We know today that there have been non organic processes associated for instance with the sulphur cycle in that most recent period. One fact has been determined is that the enzyme Rubisco, originally from the cyanobacteria nearly 3 billion years ago, is still used by plants today. https://en.wikipedia.org/wiki/RuBisCO
-
Theory of Computation
+1 for more patience than I have.
-
Great Oxygenation Event: MIT Scientists’ New Hypothesis
Good points. +1 Especially as the original posted source website is one that shows dangerous, possibly illegal, crap like this on the same page.
-
How can information (Shannon) entropy decrease ?
Despite the fact that this thread is primarily about entropy, not information, I think a digression about the difference between data and information is worth noting. Data can contain other things, besides information. In communications theory and encryption theory this might be padding data. Interestingly may padding contain additional information that is not part of the 'message' or 'information content' of the message. This is because it is possible to analyse the padding to deduce the proprotion of padding and therefore isolate the information bits. Further in computing theory data may also contain a further section, know as a 'key', for the purpose of information storage and retrieval. The persistent incivility has been reported.
-
Why is there less talk about medicinal herbs, herbal medicines , herbs , mushrooms in west today
There were formulas, but the significance to medicine of those formulas to medicine were not yet known; all the dots had not yet been connected. (PS - they still haven't. modern medicine is still a work in progress, building new knowledge on old.) Okay, so what medical knowledge did every western industrial nation eradicate between 1760 and 1840? Did Dalton ignore all of the research into heredity that had gone before? Did he dismiss all of the earlier researches into light and optics? Did he consider all foregoing studies of anatomy as too primitive to bother with? Before the later part of the 19th century chemical formulae wer recipes or prescriptions, they did not have the form we understand today. Neither correct atomic and molecular weights were available before 1850 (following the death of (Berzelius in 1848), nor was the concept of valency properly establish until 1865 (Hofmann). Valency was also confused with 'chemical affinity'. There is a fascinating account of the story of both the successes and failures during the development of this in a book by a one time oganic and pharmaceutical chemist, now a lecturer at London University and also the editor of The dictionary of Natural Products, John Buckingham. Chasing the Molecule : Sutton Publications 2004.
-
Maths problem
It is worth noting and remembering the difference between two derived quantities that sound almost the same. molarity and molality Not quite. 1 part per thousand of solution. So 1 g salt in 999 g water. Since the solution is very dilute the difference is small, but still important. Osmolarity is different again Edit Sleep well and I hope it resolves soon, without any serious effects.
-
General Relativity: Flamm's Paraboloid...
Why have you got 2 threads on this one subject ?
-
Why is there less talk about medicinal herbs, herbal medicines , herbs , mushrooms in west today
I already did, but you chose to reply about something different. Incidentally the first use of light therapy was probably the first stone age man to 'sit in the sun' to get better. The 1903 Nobel for Medicine went to Dr Niels Finsen for attempting to use 'concentrated light' - a special light source - to cure smallpox and TB. However none of these had access to lasers. In the 1960s Mester in Hungary was attempting to cure carcinomas with a forerunner of laser heat therapy. At the same time NASA was investigating the safety of lasers for non medical reasons. Their breakthrough that non-heat type lasers had therapeutical effects was not widely reported until 2001. This was the origination of PMB. PMB does not 'cure' anything it works entirely differently in ways we now understand. A pharmaceutical analog would be the new drugs that have been 'engineered in the last 15 or so years following out greater understanding of human biochemisty, such as ibrutinib. Edit I would like to add a note to those who seem to have the idea that drugs or other treatments have the same effect on anybody and everybody. This is not the case. I don't know of any treatment that works universally. So we cannot say that drug A works but herb B does not, only that drug A works for some people (perhaps a wider range of people) than herb B. Side effects are a good way to demonstrate this. My wife likes kiwi fruit, but they make her lips swell so she can't eat them. I am allergic to penecillin, but it cures many other people of many other conditions. Ibrutinib is only of use to certain genetic types (apparantly) And so on.