Everything posted by studiot
-
The Official JOKES SECTION :)
-
How can information (Shannon) entropy decrease ?
I'm sorry but you are either being disingenuous here or mistaken. You specifically related information to encoding by the use of the word 'choice' , which I highlighted from your post. Information exists, regardless of the method of encoding or even whether it is encoded or not. Furthermore the more complex examples we are discussing in this thread demonstrates that there is even more to information than this. Entanglement brings yet another layer. Information is most definitely not the choice of encoding since otherwise there would be an infinite amount of information since there is an infinite count of different ways of encoding even a simple binary piece of information. Since the choice is infinite, the information is infinite, by your definition.
-
A question for the smarty pants.
That is true and explained in my last post. When I originally saw this title , I decided to go no further. Subsequently I read the first reply when I saw it was by exchemist. And yes, I thought he dealt pretty well with the chemistry and other practicalities.
-
How can information (Shannon) entropy decrease ?
I disagree. Information is the meaning of the coding.
-
A question for the smarty pants.
Perhaps we should go for a reset here ? The actual thread topic / question is a very reasonable one put in a very reasonable way. And you did the right thing in asking if your idea was viable. Unfortunately the title included a somewhat perjorative term that clearly antagonised other members. Setting that aside, it is not unreasonable for a lay person with some scientific interest to have picked up some idea that uranium metal is fairly unreactive as is lead and so propose it for electrodes. Unfortunately the chemistry is against this proposal. The electrodes in electrochemical cells come in two varieties. Those which take part in the chemical reactions and those which don't and are merely the to provide electrical contact. In the traditional lead-acid battery the electrodes are of the first type and are not both pure metal as the underlined passage notes in the first attachment. Lead oxide is the actual chemical involved at one electrode. The attachment also gives outline working of the chemistry of the cell along with resultant cell voltages, around a useful couple of volts per cell. This should be compared with the second attachment for uranium. One thing about chemical reactions is that they have to be not only energetically and chemically feasible, they have to be fast enough at working temperatures to be useful. I have underlined the appropriate uranium reactions which are noted to be very slow and high temperature. Also note that uranium has many more oxidation states than lead, which leads to undesirable potential side reactions. It would be difficult to get a simple reaction to obtain useful output voltage reliably, as can be seen from the oxidation voltage diagram. This really is off-topic but I do wonder if you have misheard or misremembered what was said. Perhaps the scientist said, or meant but was not clear, that the sound was generated by a chemical reaction? Here is another example of 'only part of the story' and I thank swansont for this as I did not realise about the dust so I would say +1 if he did not already have too many plus points. So thank you to the 'expert' on that topic. Thanks also to exchemist for his work in debunking John Hutchinson. +1
-
How can information (Shannon) entropy decrease ?
Here is another twist to through into the data/information/entropy mix. Anyons. These are currently subject to intense study as candidates for memory.storage in quantum computers. https://en.wikipedia.org/wiki/Anyon The existence of quasiparticles called anyons was confirmed in 2020.
-
How can information (Shannon) entropy decrease ?
I am not sure. For instance when decrypting an encrypted message or expanding some compressed file, does that affect the situation? Thanks for the reply. I don't see that subsequent decrypting or expansion is relevant. Here is an example of what I mean. A list of books on the top shelf of my bookcase is information. However such as list may or may not actually be drawn up or exist. Yet the information exists and is still available and could be obtained by looking along the shelf. Even if the list is drawn up, say by taking photographs, it may never actually be read. So neither the drawing up of the information nor the subsequent processing (reading) is necessary to the existence of the information itself. The information exists, because the shelf of books exists.
-
What would happen to space if passage of time was accelerating? Equality principle. Similarity of empty space. A Shrinking matter theory that might actually work.
Neutrons also obey Pauli, as do some other fermions. https://byjus.com/jee/pauli-exclusion-principle/ That's exactly what it doesn't mean. Place two clocks at each location. Now reset one clock from each location to zero together. After some period of time is the reading difference on the faster clock exactly 20 times the reading difference on the slower clock ? Hint it cannot be.
-
The EM Spectrum: at ultra short wavelengths and high frequencies might we observe the fundamental particles of matter?
joigus actually said colour charges not changes. Here is a short extract from nobel physicist Frank Wilczek recent book, Fundamentals, ten key to reality.
-
2019/2020 Catastrophic bushfires: 2022 Worst east coast floods in memory.
Interesting idea +1 But why stop at government buyout ? Other bodies can also be involved, and are in the UK. On the other hand in the UK, there has been a debate for a couple of decades now about the folly of the authorities, not just permitting, but actively encouraging development on flood plains. I was told today that the storm surge up the river Medway reached 1.7 metres above the predicted storm surge, causing substantial damage and flooding in North Kent. This would not have happened if the Thames Barrier has not been closed, so surge water that would normally have reached far up the Thames was prevented fro doing so and flooded the Medway area instead. On the other hand, the Dutch authorities seem to manage things pretty well. Finally, have your read the book The Attacking Ocean, by Professor Brian Fagan, on the historic follies of trying to hold back the floods ?
-
How can information (Shannon) entropy decrease ?
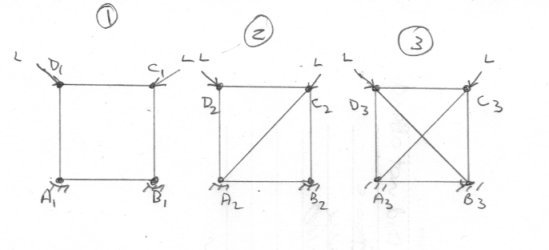
@joigus and @Ghideon Thank you for your further thoughts. I'm not sure about the role of storing the information or what difference it makes. Surely the situation simply depends upon whether such information is available or not in the system, rather than whether it is strored or retrieved somewhere ? Here is one of mine. Here are three pin jointed frames with symmetric loads, L, mounted on a foundation AB. 1) Has insufficient information to determine the forces in the frame. 2) Has exactly the right amount of information to determine the forces in the frame. 3) Has too much information to determine the forces in the frame. As far as I can tell, there is zero thermodynamic energy associated with state change here, yet it is interesting so note effects of both too much and too little inforamation.
-
The EM Spectrum: at ultra short wavelengths and high frequencies might we observe the fundamental particles of matter?
A small point, but the Ultra Short waveband is far to low in frequency or long in wavelength. This site has a more comprehsive list than yours , but uses frequency not wavelength to distinguish. https://terasense.com/terahertz-technology/radio-frequency-bands/
-
What would happen to space if passage of time was accelerating? Equality principle. Similarity of empty space. A Shrinking matter theory that might actually work.
Thank you for your thoughts. I am going to say +1. Not because I think you are right, but because you are trying so very hard and holding what I consider to be a proper discussion. OK where am I going ? Consider this: Let us consider time t' inside the spaceship and t outside. Now let us consider when t is zero. Why should t' be also zero ? Zero is after all an arbitrary point in time t when we start the timing clock. For instance t must have been running long before our thought experiment say 10,000 hours, perhaps forever. So when we reset the t clock to zero when t reads 10,000, What does the t' clock read ? If both clocks were running at the same rate then the t' clock reads (t + h) hours where h is a constant difference btween them. What now happens if you also apply the condition that t'/t = 20 , because the factor of 20 cannot be applied to the starting difference, h.
-
What would happen to space if passage of time was accelerating? Equality principle. Similarity of empty space. A Shrinking matter theory that might actually work.
Let us stop right there, because there is your problem in a nutshell. Why is it 20 times ? Why not 20.000000001 or 19.99999999999 ? How does any observer determine when it is 20 times ?, because according to your hypothersis, this factor is continually changing.
-
What would happen to space if passage of time was accelerating? Equality principle. Similarity of empty space. A Shrinking matter theory that might actually work.
I know what you are trying to say, my problem is that that which you are trying to say is either self contradictory or contains a hidden 'universal time'. Hiding it in bad mathematics does not make any difference. "One hour inside the box runs out in 30 minutes in the perspective of outside observer, so the time rate is 2 times faster" One hour according to whom ? if you say one hour in the box is equivalent to 1/2 hour outside, or the other way round, that is a transformation not a proportion from one point of view to another. If you say that some observer, who is neither in the box or in the surrounding space finds that he observes one of his hours to last one hour inside the box and 1/2 hour in the surrounding space you have 3 observers not 2. This introduction of an underlying 'absolute time or absolute space is a basic misconception about relativity that founders many an attempt to describe it.
-
Analytical Reference Standards
Every scientific discipline has its own reference standards. From your background picture, can I assume you are looking for chemical reference standards ? If so I suggest you ask a moderator to move this from 'other sciences' to chemistry. Please also provide more information as even within the one discipline of chemistry the field of standards is very wide indeed.
-
Volcanic flows...
I agree that indiscriminate use of the word 'chamber' can lead to false impression. However how big is a chamber ? For instance how big is a vacuole in an amoeba? Is that not. technically a chamber ? And are we not a technical site here ?
-
What would happen to space if passage of time was accelerating? Equality principle. Similarity of empty space. A Shrinking matter theory that might actually work.
Changes with respect to what ? The problem as I see it is that you are offering a rate of change with respect to itself, which is meaningless. using dt'/dt means the change of t' with respect to t. How can this be proportional or anything else ? That is stating a functional relationship which require a common standard to compare by. The simple relationship "distance is proportional to time" has two variables. You only have one.
-
Maybe someone can help me solve this task, I really need help, please reply
Well Captain Black chose the lengths that were given as the same to build his replica triangle on. So have you tried doing that with your two equal lengths ?
-
Maybe someone can help me solve this task, I really need help, please reply
Well spotted +1
-
Surface waves in a liquid
Yes there are plenty of youtube vids of this phenomenon about. Alternatively here is a pdf of an MIT thesis on the mathematics of the phenomenon, plus a full modern laboratory investigation Note the glass deflects, just as I said. https://www.demetraskl.com/pdf/final.pdf I don't seem to have a reply to my comment on Hess's law yet.
-
How can information (Shannon) entropy decrease ?
Good point Another good point , 'Erasure' is not a feature of thermodymic systems, that I am aware of. Most especially isolated ones since erasure can surely only be effected by an external agent, which by definition in an isolated system does not exist. Your whole post represents some great new thinking, I have just picked out a couple of points to add +1 to. +1 also to Ghideon for his thoughts and colour scheme diagrams.
-
Maybe someone can help me solve this task, I really need help, please reply
You have to use a formula that connects angles and lengths. Isoceles/equilateral triangles are the only ones that do this.
-
Maybe someone can help me solve this task, I really need help, please reply
Only by direct measurement with a protractor or other measuring equipment.
-
Maybe someone can help me solve this task, I really need help, please reply
When I said this I did not construct auxiliary triangles. I did try dropping perpendiculars from T, etc, but very quickly came to the conclusion that this would work but actually involve more work as you do not know the actual length of ST and DV. Therfore this length must cancel out and there are no geometrical theorems where this happens so trigonometric formulae must be involved for calculation. However since the actual length if ST does not matter, the result must be true for any length of ST < SV. So I just chose 3 quite different lengths and drew the figure based on these three quick sketches. Then I measured X in each case and confirmed that it was the same angle.