Everything posted by studiot
-
Zero is a number, and the big bang proves it.
Quite so. In fact it is a member of many sets, for example the integers, the rationals, the set of all even numbers, the set of all squares of numbers.................. It is also the 'additive inverse' in set theory axioms for instance in peano's axioms of arithmetic. But I was giving the OP the benefit of the doubt as they say, as to what he was really trying to say, rather than just telling him he was wrong. Especially not to offer incorrect information.
-
Zero is a number, and the big bang proves it.
How do you expect me to clarify it and why do you think I said ? I also copied a short quote to explain why I wondered if the OP meant imaginary when he said virtual. I will repeat that quote in case you did not read it. Look for the phrase 'unusual terminology'.
-
Zero is a number, and the big bang proves it.
If you don't know 'whatever it means', how can you declare that zero is not a virtual number ? Zero is, in fact, a valid and necessary member of the set of imaginary numbers, which may be what the OP means. I have already asked for clarification I also note there are some other inconsistencies in the rest of your post that need addressing.
-
Experiments and information
Kirchhoff (with two h's and two f's) was a 'polymath', who also contributed to Chemistry, particularly in the domain of chemical thermodynamics. And yes there is such a law, though it was not due to K but a gentleman named Hess. Hesse's Law and Hess Cycles. https://en.wikipedia.org/wiki/Hess's_law +1 for interesting developments of your musings. I have also mentioned them in my thread on entropy and information.
-
How can information (Shannon) entropy decrease ?
No it isn't. This is where my RAM example comes in. The information may be set but unavailable, which has the same effect as being reset. This would be an example of the Caratheodory version of the second law. "In any system, there are nearby local states that are unavailable to the system" A computation that involves no energy is the topological transformation of a square to a circle. The information is available whether the actual transformation is performed or not and whether the necessary topological theory has ever been conceived, let alone enacted. You have now also mentioned this idea Thank you for spelling all that out in detail +1 My apologies, I didn't lin to the originating thread. https://www.scienceforums.net/topic/126656-experiments-and-information/?tab=comments#comment-1200346
-
How can information (Shannon) entropy decrease ?
I like the first part. But this part runs into the difficulty I mentioned with the RAM chip. Notably the different erasure methods. For instance I can simply switch off the power to that chip. Or I can cut one or more address lines making the given cell unavailable. Or I can perform the more conventional activation of the appropriate address lines and then overwrite a zero into the given cell. I am sure there are more options.
-
Surface waves in a liquid
Yes this is a damped oscillation, as you say, but there is more going on that. I'm not sure what part of my post you were responding to though.
-
How can information (Shannon) entropy decrease ?
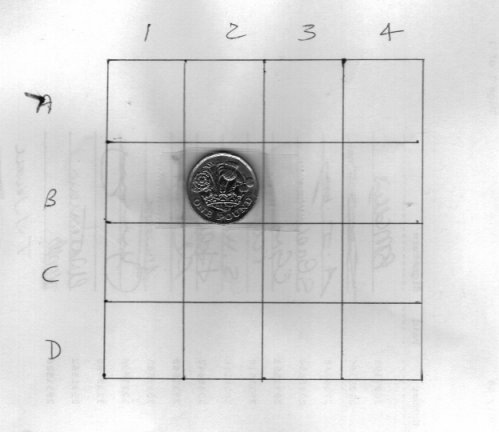
OK, thanks for the replies. Here is my process example for idscussion. I have a 4 x 4 square board as in the diagram. On one square I have placed a coin but you cannot see it (you can see the one in the diagram for the purposes of explanation) In order to 'win' the coin you ask questions that may only be answered yes or no, represented by 1 (yes) and 0 (no) When you think you have determined the position of the coin you represent the answers to your questions by a string of ones and zeros. Using the example Is it in the first column? - No Is it in the second column? - Yes Is it in the first row? - No Is it in the second row? - Yes represented as information by the string 0101 What happens to the information entropy at each stage of the Q&A ?
-
How can information (Shannon) entropy decrease ?
The thread is inspired by a claim in a recent thread that information entropy, as defined by Shannon, is the same as the thermodynamic definition of entropy which obeys the Second Law and therefore cannot decrease. Is this correct ?
-
Surface waves in a liquid
I agree there is sometimes no bulk flow of matter. But how much momentum flows in the case of a standing wave ? This is not as I understand the mechanism
-
Experiments and information
By all means demonstrate that this is the case but consider this So let us consider computer memory chip, A RAM chip. So I agree it takes a specific amount of energy input to 'write' to one cell of the Ram chip. But there are several ways to effect erasure, each with their own energy cost. Note I have started a new thread, specifically to examine any correspondence between Shannon entropy and Thermodynamic entropy.
-
Surface waves in a liquid
The disturbing force has nothing to do with gravity.
-
Surface waves in a liquid
Thank you. +1 Yes was beginning to wonder if anyone else knew how the original effect was generated. But, @Ken Fabian the distrubing force is not of the same type as the restoring force in the original effect. In fact disturbing force is generated by elastic flexing of the wall of the glass. This results in a direct ( and local) contact force on water. The waves are generated by the rotating finger on the glass, in contrast to the equilibrium tide in the Earth's oceans I referred to. Again the rotation of the Moon around the Earth provides the required periodicity. You are correct in saying that the restoring forces is gravity in both cases. Incidentally probably the best definition of an elastic system is "A system in which the displacement is directly proportional to the applied disturbing force." This would include both the volumetric compression of gases and the distortion of solids and the displacement of liquids.
-
Experiments and information
There are lots of physical processes that are characterised by the expression A = Blogp(C) Where A and C are variables and B is a constant (which may incorporate a negative sign) and p is a given base for the logarithm. Why are folks so determined to dream up a physical link beteen two such processes, one in thermodynamics and one in Information theory ? Should we be adding say a link to chemical pH ?
-
Surface waves in a liquid
It is worth, as I suggested, considering the mechanism of disturbing and the restoring forces of the original example. I agree How do you think this applies to the disturbing and restoring forces in the glass of water ? Another similar wave, but with differences, is the wave that is called 'the equilibrium tide' for the Earth-Moon system.
-
Surface waves in a liquid
Good question as you are considering the fundamentals of the question "what is a wave" ? I has been posited here that in order to have a wave a 'restoring force' is required. Surely this force is only required if mass is involved. If you are going to stick to the original example, mass is involved so it is worth investigating disturbing and restoring forces. So thank you for starting off a worthwhile discussion, but please try to offer more focus at the outset.
-
Gravitational Potential Energy in a 2 dimensional Universe
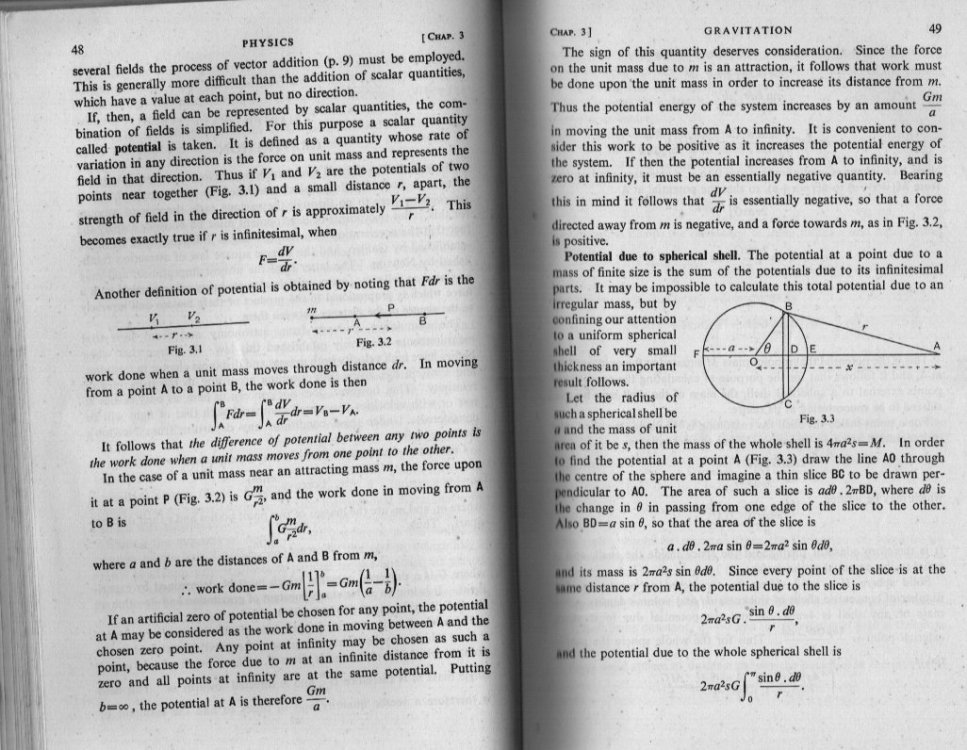
I think you need more black coffee. Was he not only following your lead? Since you don't want to discuss the basics with me I will leave you with this 1950s University Physics textbook extract, which directly addresses what seem to be you basic misunderstandings. In particular it provides a simple mathematical derivation of the 1/r relationship from the 1/r2 Law. Please be aware this applies in any number of dimensions for Newtonian gravity. Also note the explanation about infinity and the negativity of PE and the difference between PE and potential. As regards the reasons for our universe being a 3 + 1 universe: Vector statics is complete in a 3 spatial universe and vector dynamics complete in a 3 +1 universe, but not a 2 spatial universe or a 2 + 1 universe. As I have been trying to lead you towards. In the early days of Einstinian Relativity, there was much discussion about the possibility of other universes with A spatial and B time dimensions and the consequences if these were assumed. Eddington provides a good mathematical reasoning to discount these for our own in his book 'The Mathematical Theory of Relativity'.
-
Experiments and information
Perhaps you have no takers, because, like myself, others are confused as to where you are coming from or going to with this question. Please clarify.
-
The Science Of Stupidity.
Somewhere between a quarter and three quarters of the world's population suffer this. Is that good/bad? Thank you for your reply. I made that comment not realising that you might have meant something totally different from what I understood your statement to mean. That is why I subsequently asked you to clarify it. I still don't know what you meant. Only a few agree is quite clear, but what they agree to is not. Do you mean that they are prevented in some manner from speaking whatever they want or do you mean that they should not speak whatever they want (common colloquial use of can't) ?
-
Gravitational Potential Energy in a 2 dimensional Universe
You have made some very valid points in this thread, but I believe Vashta is talking about our Universe. Further the rules of SF require our Universe as this was posted in classical Physics. I agree, but would go further and suggest that GR is inappropriate in this thread, except as a passing mention. +1 Incidentally Markus said solve Laplace, not deduce it. I have plenty of expositions of Newtonian gravity involving Laplace, I just want to help Vashta find the appropriate format. Of course solving Laplace will not get us the potential. It will get us the potential function, which is different. It is confusing to those just studying this subject that the word 'potential' is used in several different ways. I actually must now apologise and correct an incorrect statement I made earlier about the units. I said that potential energy and potential (difference) have the same units. This is not quite correct. PE has units of energy, potential by itself or PD has units energy per unit mass.
-
Zero is a number, and the big bang proves it.
You have placed your thread in the Mathematics section, which is totally independent of Physical Science, as are the respective disciplines definition and use of the concept of zero. Please clarify what answers/discussion you are seeking. You are correct in stating (in the title) that in Mathematics Zero (by itself) is a number. I note that your 'absolute zero', 'virtual zero', polar opposite, etc hint at mathematical set theory which leads to a mathematical notion of zero, but using unusual terminology.
-
The Science Of Stupidity.
Why don't you just clarify your originally ambiguous statement ? Who are the few and what do they agree with ?
-
Gravitational Potential Energy in a 2 dimensional Universe
Actually none of this is a solution to Newton's Law of Gravity. Newton did not use potential theory - It had not been invented in his day. In modern notation Newton's Law of gravity is [math]F = G\frac{{Mm}}{{{r^2}}}[/math] This is a straighforward algebraic expression and a solution means that you have a value for all the variables, except one so you substitute them into the expression to obtain the unknown quantity. So instead of arguing at cross purposes, Vashta how about posting your maths working to obtain whatever you have obtained ? Then we can help you tighten up your maths. As regards the curl, do you understand the concept of a vector that represents a turning moment ? I am convinced that this is the key to your difficulty reconciling 2D and 3D. Actually
-
The Science Of Stupidity.
You mean I can't blow my own trumpet ? But Zap, I love Daleks.
-
Gravitational Potential Energy in a 2 dimensional Universe
No, I was keeping in mind this is a discussion about Classical Physics. Working in terms of densities - something per metre, something per square metre, something per cubic metre is a very common technique. Of course if you go to two or three dimensions your integrals become area or volume integrals, which is why I keep recommending using the one dimensional case for starters. How about answering my questions, they are designed to help ?