Everything posted by studiot
-
What does the ‘infinite monkey theorem’ suggest about the anthropic principle?
Yes the authors are quite correct in what they say about the scenario they describe. But it would be statistically quite wrong to apply this statement to evolution. The monkeys are performing the same (statistical) experiment over and over again. That is they are repeating one single experiment. Evolution is about the confluence of many many simultaneous experiments. The statistics of such a process is entirely different. Applied to the monkeys this is equivalent to applying a whole bunch of 'filters' or constraints, themselves perhaps random in some way but maybe also biased. So that for example certain keys are occasionally electrified so the monkey will shy away from them. And another filter is applied so that the resultant electrification shepherds the monkeys into typing the keys for the letters in their order of frequency in the English language. Now apply a very large number of such filters all compounded together. I wish you well in finding out the resultant text the monkeys migh come up with as the possibilities are truly staggering. So much so that you could be studying for the age of the Universe and never see a repeated text.
-
Free alkaline & alkali ions in water
Look around you. Water, alcohol, oil, resins, methane, nitrogen, oxygen, carbondioxide, benzene; These are common liquids or gases. And they are all covalently bonded, albeit some have straightforward covalency some have dative covalency (ie are polar). Compare this with sodium chloride, iron oxide, copper sulphate; These are all common solids. And they are all ionically bonded. This situation represents the very large majority of cases. This is no accident, there are good reasons for this. That is to observe that ionic compounds tend to from solids whilst covalent compounds often appear as fluids. Yes one of these reasons is molecular weight. But there are plenty of examples of ionic solids with a lower molecular weight than covalent liquids, eg sodium chloride is 58 whilst benzene is 78, both from my list. So it is instructive to consider what is different. The difference is that in a fluid the molecules have a degree of autonomy not present in a solid. They can move about as a molecule. And most important you can for instance identify one particular carbon atom with two particular oxygen atoms forming the 'molecule'. However you cannot identify a particular sodium atom (ion) with one particular chlorine atom (ion) in the solid. In fact electric forces link one (each) sodium+ to 6 chloride- The coordination number is said to be 6. So the intensity of the charge difference is distributed that way. https://courses.lumenlearning.com/cheminter/chapter/ionic-crystal-structures/ This is the key difference of importance to your question. The chemical implications of these can be very complex indeed as both John Cuthber and exchemist are trying to tell you.
-
Help with calculating the number of milligrams present in the solution
As I see it, @popcornfrenzy has not yet told us the entire question, as it is writen in the book or wherever. How do we know that we have to calculate a number of milligrams ? This was nowhere stated in the supposed complete copy of the question. I have suggested it is of the sort in my attachment, starred to show how common this practice is. So yet again I ask for the full question. @popcornfrenzy If you are not confident with these, there are many books of nothing but practice (drill) questions with answers. Practice makes perfect. And we do want our Baristas and Pharmacists to give us the right cocktail every time don't we ? 🙂
-
Finding pH of water
From what you have said before, I would think the final objective might be to help you understand the following presentation about dilution and pH calculation in swimming pools. https://courses.lumenlearning.com/cheminter/chapter/calculating-ph-of-salt-solutions/
-
Help with calculating the number of milligrams present in the solution
It looks to me like the sort of 'question' you find in texts on Pharmacy or Pharmaceutical calculations. Very often there is a general question such as "For each of the following mixtures of 100 mL of each, calculate the number of milligrams of each ion present in the solution mixture" This is followed by a list of drill questions with different solutions. By itself it is incomplete. 'pf' or percentage fraction is generally used by Pharmacists to mean grammes per litre or g/L and should be writen 0.4% which means it contains 4 grammes per litre of solution. For example see here https://www.medicines.org.uk/emc/product/1869/ I think the important issue with this question is that your strengths are in different units so you must convert to one or the other to obtain the concentrations in the resulting mixture.
-
Help with calculating the number of milligrams present in the solution
This is not a question Nor is it a complete statement. So first you need to get is a full and accurate statement of the question and the values of the concentrations concerned.
-
Does Maintaining pH Require Energy?
Different parts of the body operate at different pH values. Some also operate a variable pH values for instance there are literally thousands of catalysed body processes many of which are pH sensitive. So the first question is What do you mean by the body pH ? The body processes alkaline foods in the stomach with stomach acids and further in in the digestive system it processes alkaline foods. This I understand is the basis of the 'Hay Diet' The little experience of this I have seen in others, is that dieting works by reducing calorie intake eg substituting cabbage for potatoes, rather than any pH control. https://en.wikipedia.org/wiki/Hay_diet
-
Finding pH of water
A solution is a mixture of two or more substances. Just to consider two, A and B - alcohol and water or salt and water. The mixture contains a certain % of A and (100 - %A) of B Adding (pure) A or B to the mixture will increase the concentration of A or B, decreasing or diluting the concentration of the other. In other words diluting is the opposite of concentrating. I know in common parlance we often use diluting to mean to add water. This is not untrue just only part of the full (scientific) story.
-
Finding pH of water
Do try to do the short question I asked at the end about common salt. It is meant to help develop your understanding.
-
Finding pH of water
Science in general does not do 'proofs' - That is for Mathematicians and Lawyers, although their definitions of the word are somewhat different. Science does hypotheses and deductions, which can tested against observations. But it should always be open to modification following further observations which show something different.
-
Finding pH of water
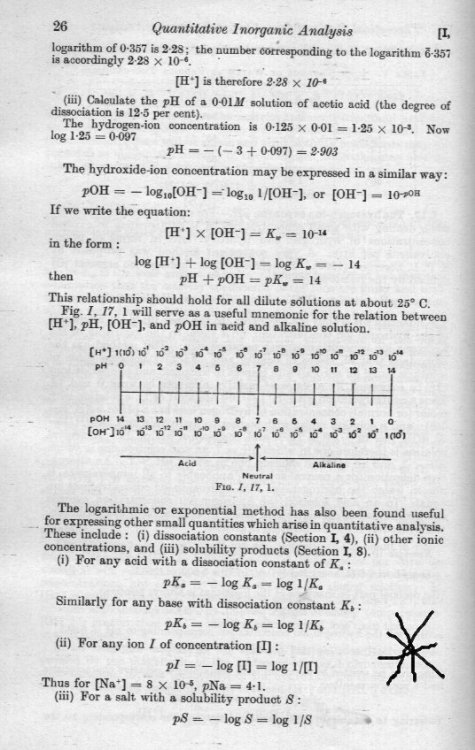
It doesn't change anything, it is not a proof. It is a derivation to show where it comes from. It also shows that you are correct a little bit of information is lost in the derivation process and it is a matter of convention which way up we define the fraction. This is an arbitrary choice that is internationally adopted and must be simple remembered. I think I made the comment that remembering which way up trips many students up so stressed this point. The convention adopted does has the advantage that these constants are very small (much less than 1). So this makes the pH and pX equations fit neatly into a convenient range of numbers. When you start to introduce other substances to the pure water this changes the pH and other constants become involved. I have starred the comment in the attachment below. So it becomes even more important to get the fractions the right way up. Here is a very simple calculation for the pH of 0.03M HCl in pure water. Here you also need to know that HCl is a 'strong acid' This means that it is totally dissociated in water. So the concentration from the hydrogen chloride of H+ ions = concentraction of Cl- ions = 3 x 10-2M We know that the concentration of H+ ions from the water is 1 x 10-7M So the total concentration of H+ ions is (0.03 + 0.0000001)M = .0300001M So we ignore the 0.0000001M from the water. So the log10 of 0.03 = log (3) + log (10-2) = 0.48 + (-2.0) = -1.52 So the pH of 0.03 HCL is -(-1.52) = +1.52 This is the simplest calculation and shows what happens when either a source/sink of H+ or OH- ions is added. In this case the OH- concentraction is unaffected since Cl- ions are added. Note that this solution is once again electrically neutral (contains the same number of + and - charges) and at chemical equilibrium. So if you added 36.5 x.03 = 1.1 grammes of HCl to every litre of pure water the pH would be 1.52 So as the simplest possible exercise can you predict what would happen to the pH of pure water if you added 5.84 grammes of sodium chloride (NaCl or common salt) to pure water ? Then the solution would not be in chemical equilibrium and this means that the concentrations would be changing over time.
-
Simple yet interesting.
Actually I have come across something connected to agebraic geometry and group theory. I have just been trying to remember it. But not to vectors. You require a whole lot of extra mathematical structure for vectors. I certainly think that is the wrong tree to bark up. Look at it like this 15 = 5 x 3 ie it factorises into 5 and 3. But 15, 5 and 3 are all numbers (integers to boot). That is they are all the same kind of (mathematical) object from the same set. This is a consequence of and consistent with the axiom of multiplication that for every a, b in the set a x b = c is also in the set. However this is not generally true for vectors as it would require the product of two vectors to be a vector in the same set. In you case you have talked of the vectors ' 5 and 17 in the plane so the product (whatever it is) must also be a vector in the same plane, which the vector cross product does not give you. Neither does the vector dot product.
-
Einstein translated in terms of tau (2π)
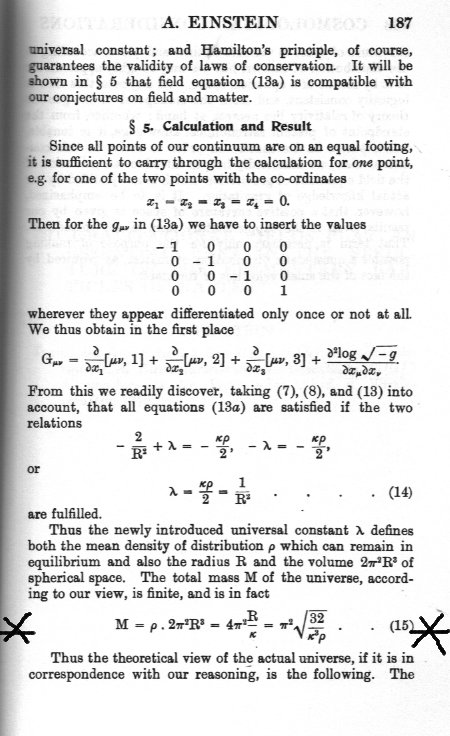
Some of them are indeed oversimplifications. They are not always valid and do not necessarily lead to a conservation law. However since you refuse to answer my question here are the words of your guru on the subject I asked you about. So have you ever met the half-side of a cube ? Of course one half-side times another half-side (of a cube) gives you the area of a quarter side Whereas A whole side times a whole side gives the area of a whole side. Much more pleasing, yes ? Is there something wrong with discussing radii and diameters ?
-
Millikan's oil drop experiment
Sorry I wasn't completely clear. The thing is that 'Millikan's Experiment' was not one single experiment at all. It was a determined effort by Millikan to measure several important properties of 'the electron' over several experiments during the years from 1909 to 1913. The results and conclusions of this work were first published Phil. Mag., 34, p1, 1917 and later in a series of books which started out with the title The Electron and was revised a couple of times to The electron: its isolation and measurement and the determination of some of its properties. (1919) and later editions Electrons (+ and -), Protons, Photons, Neutrons, Mesotrons and Cosmic Rays (1936) As a result of this work and also work on the photoelectric effect Millikan was awarded the 1923 Nobel prize. The result of this ongoing work over an extended time period, during which other people also added discoveries has resulted in variations in modern accounts in more modern texts. However the importance of this work is that it enabled the drawing together of several branches of Physics and Chemistry towards the more coherent whole we have today. Before Millikan, Faraday had discovered the laws of electrolysis and Avogadro had presented his hypothesis, both in the 1830s. Then, however the molecule was not at all established, Dalton's atoms were still on pretty shaky ground and ions and ionisation were yet to come. Between then and the late 1800s the particulate nature of matter became more and more established, but electricity was seen as quite a different subject. The idea that the particles of matter were held together by electric forces was yet to arrive. Electricity was known to come in two polarities, positive and negative, but details were not known. Then in 1897 Thomson discoverd 'particles' of electricity. He had discovered the electron. Furthermore he measured the ratio of the charge to mass, e/m for this particle. Then in 1909 Perrin came up with a good value for the Avogadro constant or number. That is the number of particles in a mole. It was seen that this tied in with Faraday's work since 96500 coulombs were required to deposit 1 mole of a monovalent element. Since the proposition was that 1 mole contained a large (Avogadro's) number if identical particles it followed that an identical charge must be supplied to deposit each one and that these might be tied in with Thomson's electrons. Thomson and independently Wilson were experimenting with the production of ions in gases and measuring their charge, both positive and negative. (This part is not normally taught in chool Physics these days) but it was their methods that Millikan drew upon and extended so that: It was at this point that Millikan entered with his series of experiments that were able to determine not only the values of both the mass and charge on the electron but that the charge was equal to the 96500 coulombs divided by Avogadro's number and that it was negative. So electrons were particles that were carriers of a fixed amount of negative charge that also possessed a small amount of mass compared to any atom of any element. The way was now open for Physicists to develop atomic models and Chemists to develop electron exchange models of ions and valency (chemical bonding). Both of which developed rapidly in the early 1900s. In his actual experiments Millikan changed Wilson's 'condenstaion of water' method to a fine spray of oil. This fine spray did not evaporate like water and could be controlled and came ready with a small charge due to friction in the atomiser nozzle. Since this was a small charge and many droplets were not charged at all, in later experiments he followed Wilson in irradiating the air in the chamber with X rays. This first ionised some of the air and then the air particles transferred this to the droplets by collision. A swansont notes, he was able to control the potential on his plates so the he could measure for both positive and negatively charged droplets as he did not initially know which would occur. Today we sometimes use alpha rays (positive) insted of X rays. These steal electrons from the gas, creating positive gas ions, which in turn regain electrons from the oil droplets, creating positive oil droplets. A rays, being neutral will separate electrons from the gas particles, creating posotv gas ions and free electrons, some of which attach to the oil droplets forming negative ions. So Millikan's original equation was If a droplet aquires a charge q, then the resultant force on the droplet will be mg ± Eq depending upon the sign of the charge q. (E is the strength of the electric field between the plates) I assume you have an idea of the method but I can provide more detail if you like.
-
Einstein translated in terms of tau (2π)
Instead of being rude and condescending about our abilities why not just post the requested information and see if we understand it ? I note you have ignored my comment about diameters v radii. Using diameters instead of radii is equivalent to asking "what is the diameter of curvature in differential geomery ?"
-
Millikan's oil drop experiment
It's a very good and perceptive question. +1 It should be noted that the original experiment as conducted by Millikan was very different from the simplified one studied in schools and actually carried out in some of them. In particular neither Millikan nor anybody else originally knew what the charge was. The droplets from the atomiser were ionized by means of X rays. Since then it has been discovered that friction within the atomiser is sufficient to ionise the droplets and that they then carry a negative charge. Also it was not originally known that there was a unit charge, e. This actually came out in the original experiment. Millikan found that charges by balancing gravitational, frictional (viscous) and electrostatic forces on specific drops. He discovered that the charge was negative and could only be changed in integer multiples of a base unit of charge, e and the change could not be made smaller than this.
-
Finding the range of a function
This leads to put y = 2 then x2 -2x +1 =0 (x-1) (x-1) = 0 x =1.
-
Einstein translated in terms of tau (2π)
He does indeed as in the attached, but I'm pushed to find a tau in either document. If Pi was good enough for good old Albert, why is it not good enough for you ? In respect of diameter v radius, There are good practical reasons for retaining both measurements. In respect of your other commitments, you have reached your 5 posts in your first-24-hours anti-spam limit. So I suggest you use that time to consider your reply and also think very carefully about the SF rules.
-
inductive effect and hydrogen bonding
For the nitrophenols phenols look here. https://www.vedantu.com/question-answer/the-correct-order-of-decreasing-acidity-of-class-12-chemistry-cbse-5f4cb04546779f7310735ca5
-
Einstein translated in terms of tau (2π)
Yes you are right, my mistake, 2πR = τR =2πR = τD/2 = πD Why is this the most important thing to start your thesis with ?
-
Einstein translated in terms of tau (2π)
Yes I'm back and my simple question remains unanswered. Why is 2πR different from τD ?
-
Einstein translated in terms of tau (2π)
Fair enough. Now let us see this equation and please explain why there is any essential difference since the substitution of the diameter for twice the radius in calculations makes no essential difference.
-
inductive effect and hydrogen bonding
First are you sure you have the correct order of acidities ? These are similar questions to the ones you asked last July but you are correct there is an additional effect with the possibility of H bonding. Please check your acidities then study this reference and then come back with any further questions. https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/20%3A_Carboxylic_Acids_and_Nitriles/20.04%3A_Substituent_Effects_on_Acidity
-
mystery glassware identification - anyone knows what this is ?
Looks suspiciously like you nailed it. +1
-
What's the story with physics?
Don't think this guy is a kiddie. Someone with that handle has been to many debating, christian and maths sites over at least the past year and a half peddling the same stuff. here is a comment forma member on a maths site