Everything posted by studiot
-
Ionisation in radioactive decay of atoms
Yes, sorry. Quoting you instead of the OP was my mistake.
-
Plate Tectonics and New Geological Process:
Unfortunately this article is subscription only. I would like to see at least a diagram in case something is lost in the translation from Turkish.
-
Martian Hydroelectric Concept
As an obviously competent engineer I am disappointed with the obviously politician's brush off when pressed for hard detail, more especially as you invited comment. Here is the problem I am trying to reconcile. You have mentioned several different pipe sizes, and somewhere a square metre of cross section. So let us consider 1 m2 section of pipe 1 metre long, at the frozen stage. This has a volume of 1 cubic metre. Ignoring, for the moment, the small difference in density between ice and water, this has a mass of 103 kg So to calculate the approximate energy reuqirement to raise this from ice at -4 C to water at +1 C ie to melt it we require 103(4*2050 + 334000 + 4200) = 3.464 105 x 103 Joules per m3 Now the rate of insolation on the mars is 590 w/m2 or 5.9 x 102 J/m2 per second So it requires ( 3.464 x 108 ) / (5.9 x 102 ) = 6 x 105 square metres of martian surface to receive this energy every second multiplied by the rate of movement of the ice/water interface as this was calculated on a 1m/s basis and assuming perfect energy conversion.
-
Martian Hydroelectric Concept
Sadly you are still not answering my question(s)
-
Ionisation in radioactive decay of atoms
You are asking this question of the Physicists here. In most Physcis textbooks the authors are not to worried about charge so the equations presented do not generally observe conservation of charge. So the equations presented often have a beta minus or alpha positive charge on one side, but no charge on the other. So these equations do not balance in respect of charge. For example Chemists are more careful so here is an extract from a Chemistry textbooks that explains this in detail, balancing the charges as well. And yes the equations now balance for charge. Some reactants and/or products are now shown charged (eg as ions or whatever) Another way to compare is to understand that Physicists are talking about nuclear reactions and so use the chemical symbols to represent the nucleus, which always carries a positve charge equal to the atom ic number. Chemists use the same symbols to represent the electrically neutral atom so must always display the charge to refer to an ion. Does this help ?
-
Martian Hydroelectric Concept
Well perhaps you are not the only one being unclear since this post of yours describes exactly what is worrying me and I thought I had stated in my last post. Someone seems to have perhaps understood it, although your answer suggested that you did not catch it. Do you think that either Martian conditions allow either freezing or thawing at 18m/s ? That is driving along at 40mph. What sort of heat transfer coefficients are you envisaging for the pipline and what about the energy flows to accomplish this ?
-
A real development in fusion power generation or just another step on the path ?
Article about Tokomaks in Britain and America Interestingly set in the business section, not the science section of the BBC. However it does offer some useful up to date facts and figures. https://www.bbc.co.uk/news/business-56843149
-
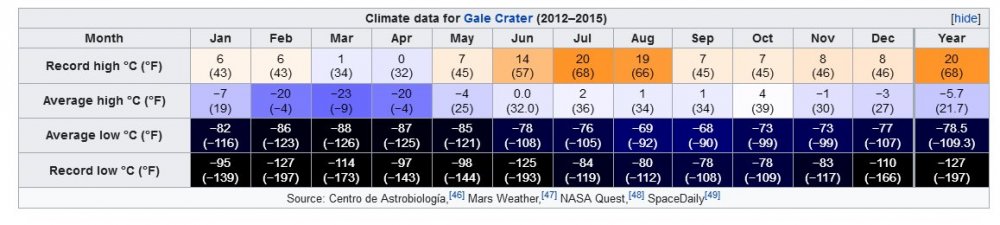
Martian Hydroelectric Concept
After checking a few figures, I agree with your starting point about the length and rotational velocity of the pipeline in rounded numbers. But exchemist has a more supportable view of the rotational velocity of the interfaces. Have you thought about how long it takes for the thawing to occur ? Here is a table of measurements from Wikipedia. Note that at this site the temperature does not rise above the freezing point of water at any time of day or night from November to May
-
Blowing hot and cold
All good points pertinent to a much more in depth analysis than my offering. +1 However it should be noticed that the effect of blowing over soup is always cooling on the soup, however you blow. But the effect of blowing over the hands is cooling or warming depending upon how you hold you hands and how you blow.
-
Electricity using low grade heat
Yes indeed it is possible to extract energy from the air. Wind turbines can turn this into electricity. Windmills or windpumps can turn this into useful work. Air pressure can be use this to raise and lower large steel pressure chambers or 'gasometers'. Heat pumps can turn this into useful heat. But none of these directly fit your description which appears to violate the First Law of Thermodynamics. Members here will happily discuss your invention and perhaps help you towards a practical revision that would actually function. I suggest you start by understanding the difference between loaded and unloaded machinery, both electrical and mechanical, and the importance of flow rate to the output of machinery.
-
Sign rule for multiplication
I note you have posted this in Linear Algebra and Group Theory, not simply general Mathematics. So I assume you know what these terms are and that Groups are connected with symmetry. Well signed multiplication is an operation with a symmetry. The full list of possibilities is positive times positive makes positive positive times negative makes negative negative times positive makes negative negative times negative makes positive (The one you asked about) As you see this list is symmetrical: there are two ways for a positve result and two ways for a negative one.
-
Understanding reaction types
Is this really a homework question or just a request for understanding of material you have been presented with in a course or read ? I ask because it looks much more like the latter and such questions can legitimately be asked in the main Chemistry sections. OK so there are several different classification schemes for chemical reactions. Each has its own point of view and the schemes are not mutually exclusive. So you first list is about reactants and products. That is what substances you start with and what substances you end up with. Say A + B = C - combination A = B + C - decomposition or dissociation AB + CD = AD + CB - replacement or elimination and so on. There are further reactions types than your first list in this viewpoint. Your second list narrows down these wider categories in some way. So Acid + Base = Salt + Water Is an elimination reaction where CB is always water and AD is always the salt. (This has been extended beyond water in more advanced Chemistry) If the reaction is in solution and AD is precipitated then this is also a precipitation reaction. So you can see some overlap in the categories beginning to appear. Oxidation is the addition of oxygen or the removal of hydrogen Reduction is the removal of oxygen or the addition of hydrogen Since combustion is one of these (which one ?) It is a redox reaction. Note that all these classification types start with substances and end with different substances. In more advanced Chemistry your will meet others views which are about what goes on during the reaction and the movement of electrons and/or protons from one substance to another. These may be called reaction mechnaism views. Where are you in your studies to have been given the first list as the basic 5 ?
-
Do virtual particles exist?
What is meant by virtual (virtually is an adverb which refers to verbs, not nouns) is my point. Yes we do understand virtual and have been using it for centuries. Does you eye see the virual image obtained through a magnifying glass ? What is the difference between a real and virtual image ? If you understand the difference between pressure and force you are using a good example of another virtual phenomenon. Consider a gas. Imagine a frame somewhere in that gas defining an area. What is the pressure across that frame and is a force acting ? The frame and its area are virtual as pressure is only exerted on the boundaries of the gas. In other words it is only realised by the interaction with the real world around it, just like the quantum virtual particle.
-
Blowing hot and cold
But this is strictly speaking off topic. I admit to discussing tea rather than soup but the principle is the same. So the topic is what is the difference between blowing on your hands and blowing on your soup (or tea). ? So you can't dismiss the subject of the topic.
-
Blowing hot and cold
Thank you for your thoughts. But I can only suggest you read very again carefully both the opening post and what I actually wrote. What you have just written is incompatible with both (and with the scientific method as well).
-
Do virtual particles exist?
Has everybody (MarkE in particular) given up on this one ?
-
Safer for a healthy 32 year old: contracting COVID or getting the vaccine?
Well I'm sorry I can't see any hostility in my response. I even suggested you do exactly that - hear and evaluate the evidence, but from the medical professionals directly concerned. Ultimately the decision is yours alone.
-
The "Ice Bomb" thermal engine
I'm not suprised if you tried to study it via Carnot cycles. That really is the hard way. Thermodynamics divides things into a system and the rest of the universe (ie the surroundings). To do this successfully it must always specify the system boundary. Having done this state variables (preferably directly measurable ones) are introduced for both the system and its surroundings. Pressure, volume and temperature being the most recognisable. Now it was found that the area under a P - V plot was equal to the mechanical work that is exchanged across the system boundary between the system and its surroundings. This leaves temperature and a similar variable was sought to pair with temperature to calculate the heat energy exchanged across the boundary. Entropy was the name given to this variable. So the area under a T - S plot is the heat energy that passes across the system boundary. Unfortunately entropy, unlike P, V and T is not directly observable so we use measured tables instead. That's all there is to it.
-
The "Ice Bomb" thermal engine
John is that rare breed of Scotsman who possesses a grand sense of humour. And of course, burn is the Scottish for a stream. The additional double entendre was unintentional and unnoticed by me, until you pointed it out. Since no large deflection is required I was thinking in terms of a diaphragm deflecting and maybe pushing a rod. The way to calculate this is not from the equations because water is an exception to the normal pattern. You need to get out a set of 'International Steam Tables' - a quick look shows mine are in the garage - and read off the entropy change on a T - S diagram to obtain the energy flows easily. I don't have a convenient glacier to effect the freezing part of the cycle or a convenient hot spring/geyser to provide the thawing, and even if I did they would still be part of the system and their entropy changes need to be included.
-
Homework help regarding force, weight , acceleration and momentum
Oh dear was my spelling really that sloppy ? 😳 Sorry all.
-
The "Ice Bomb" thermal engine
Don't you need a 'wee burn' to run a stream engine ? 🙄
-
The "Ice Bomb" thermal engine
Yes perfomance of the cycle a requirement of the second law. So another way to put it is that the work obtained from the expansion of the ice will be less than the heat required to complete the cycle and thaw the ice, ready for the beginning of the next cycle. Such situations were exactly why Maxwell, Clausius and others stated the second law in cyclic format, as I have already noted.
-
Homework help regarding force, weight , acceleration and momentum
Th The acceleration needs to be negative so that the calculation gives the correct direction to the calculated forc in the final eauation F = ma. ie the force is a retarding force.
-
Blowing hot and cold
Thermodynamics is an experimental science. The OP was doing pretty well until he got to this Not really, as a bit of simple experimentation shows. 1) Breath out through a wide open mouth and compare the thermal effect on your hands and the cup of tea. Pretty ineffective cooling on the tea and heating on the spread open hands. Slight heating on cupped hands. 2) Breath out through pursed lips Effective cooling on both the tea and the spread open hands Effective heating on cupped hands So pursing the lips is not the controlling factor to make the difference. So what is ? Well cupping the hands seems to lead to substantially increased hand heating in all cases so lets deal with this first. Cupping the hands forms a small, if leaky, chamber into which the exhaled air is drive, increasing its pressure. Work is done on the air increasing its internal energy. This work is done by the chest muscles of the body as can be felt during the exhalation. Thie work quickly degrades to heat which transfers to the chamber walls (hands) as the atmospheric pressure is reasserted. So that is what warms the hands . If you blow on the spread hands or the tea, evaporation is enhanced as already noted, which process carries heat away from the natural moisture on the hands or the surface of the tea. This occurs whether the lips are pursed or not. The difference is the cupping of the hands.
-
The "Ice Bomb" thermal engine
You have not analysed your heat engine correctly since you have missing elements. The apparatus to cause this should be included So yes If you allowed the ice to expand in a non destructive way by compressing something, this compression could be partly extracted by causing it to do useful work. But you analysis should include the work input to the freezing apparatus which will be greater than the work recoverable by the compression. Remember also that the classical second law applies to a cyclic process, and may be 'violated' in part of a cycle. A bomb is not a cyclic process.