-
Posts
18315 -
Joined
-
Last visited
-
Days Won
104
Content Type
Profiles
Forums
Events
Everything posted by studiot
-
Now here is a thermodynamic question that all the textbooks in world will nothelp you with. However, even though (climate) conditions in Lagos mean you probably never experience this problem, as an experienced enginner, you may well be able to suggest a good way forward. When casting concrete in cold weather the concrete will suffer terminal damage if allowed to freeze in the first 24 hours. Suggest countermeasures to avoid this.
-
Absolutely not. That is even more obscure than ontology. Some examples of what I mean by Analysis : 1a) I hand you a container of a a pure gas and ask you to measure the % carbon in the gas. result 83.7% 2a) I ask you to measure the contours of an existing embankment (survey it). 3a) I point to the pavement of a concrete road leading into a nuclear power station or petrochemical complex ask you to measure the stength of the pavement concrete because I need to run plant along the road safely. Examples of Synthesis 1s) I ask you to manufacture a canister of a pure gas containing 84.7% carbon 2s) I hand you a set of plans and ask you to construct an embankment according to those plans. 3s) I ask you to construct a new concrete road with strength at least 6,000 psi to accomodate the plant I need to run along it. Does this make it any clearer? In each pair of cases which do you think is the more difficult task?
-
Sounds good. Does the stress here introduce a value judgment of a methodology based in its ontological classification (ie. what name you give to it)? I'll attempt to paraphrase Feynmann "If it disagrees with experiment, it's wrong". Conversely, all methodologies that agree like-for-like with measurements of the real world must be equivalently valid. In my experience ontology carries with it many hidden traps which makes me very wary. It's a branch of metaphysics and maybe best left to the theologians to play with. Not really, no, although ontology is too airy fairy for me. I am fond of pointing out the twin complementary processess of analysis and synthesis. Synthesis is largely practised by Applied Scientists and Engineers, (following the analysis of a problem) In my opinion it is more difficult to create something that does not yet exist (a dam a motorway, a chemical plant a harbour etc) to meet a specification than to analyse something that is. already there. I am worried about this since I picture say a red hot poker being thrust into a bucket of water as a line heat source or point heat source in any section. Surely this meets the specification of your point P , but generates motion and dispersion away from P not towards it ? Maczek (as it is spelled on the fron of my copy) is a very good and clear basic book I would recommend to anybody. He does not dilly dally with microstates like some but uses partition functions to the full. What is you opinion of partition functions v microstates ? You might also like to look into what Mandl (Manchester Physics series Statistical Physics) about your youtube issue. I think (please confrim or correct) that this is a description of it in his introduction to the second law. He goes on to split the probability function into two functions by , not the states themselves, but of the size of the fluctuations as a result of the N or n. He shows how the smaller N is the larger the expected fluctuations are from 'equilibrium'. Fluctuations sizes for a single particle are 'off the scale'
-

Attempting to create a generalized graph of mathematics
studiot replied to ALine's topic in Mathematics
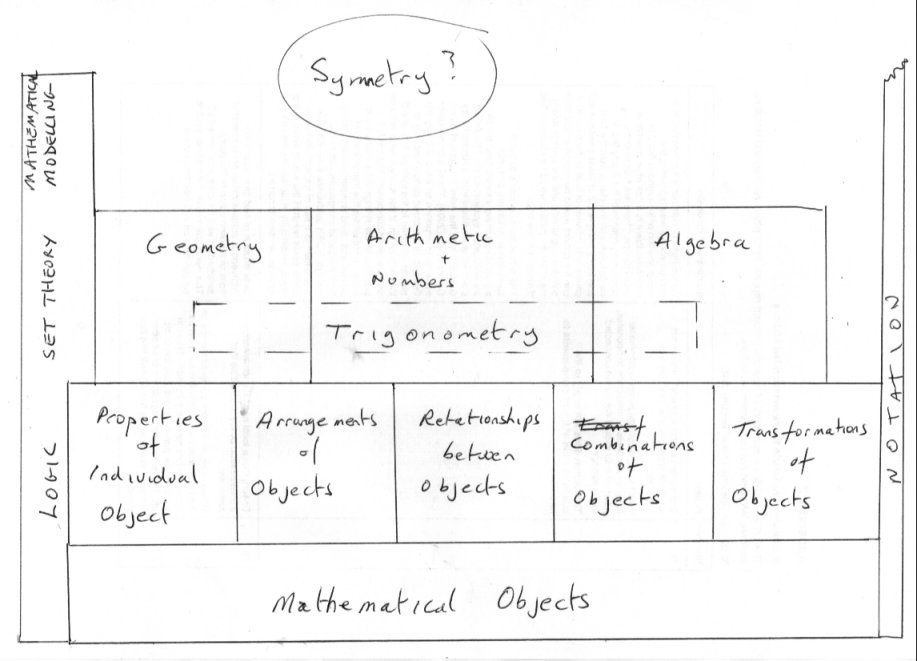
OK so here is the beginnings of my suggestion for one small corner of your map. What is Mathematics about ? What does it do? Well Mathematics is about mathematical objects and what we can do with them according to rules of logic. So what are mathematical objects ? Well they are objects like points and lines and sets and functions and shapes and angles and numbers and......... So what can mathematics do with them? Well it can describe Properties of objects such a symmetry, Arrangements such as an array (matrix object) , arranging points to make a square and so on. Relationshipes between two or more objects such as 10 > 3 Combine objects to generate another (different ) object such as 9x = iy) making a complex number Transform one object into another for some purpose eg taking logarithms Some of these operations are important when used to make the branch of maths called Geometry; Others support number theory and yet others support Algebra. So where does Trigonometry fit in? Well sinx is defined as opposite/hypotenuse and is a number so number theory comes in. Yet you can perform arithmetic operations such that sin2x + co2 x =1 so arithmetic is onvolved and again [math]\sin x = x - \frac{{{x^3}}}{{3!}} + \frac{{{x^5}}}{{5!}}...[/math] So summation of algebraic series brings in algebra How are we doing ? -

Attempting to create a generalized graph of mathematics
studiot replied to ALine's topic in Mathematics
I intend to post a diagram (it's too scruffy at the moment) later tonight. Whilst you are still online look at these links https://en.wikipedia.org/wiki/Mathematics https://en.wikipedia.org/wiki/Glossary_of_mathematics -

Spacetime Diagrams Lorentz Transform.
studiot replied to can't_think_of_a_name's topic in Relativity
I didn't say was wrong, I said it lead nowhere. Any straight line through the origin is of the form y = mx where m is the slope. So you could have written the last line straight down since 1/v is defined as the slope. But you have also added the condition that xB = 0. This can only occur at a single point as I noted above. All your last two lines show is that distance = speed x time or time = distance / speed. This is pretty common knowledge. -
Accepted let's move on. Clearly all those years of experience, plus more which must have been spent in study of the subject, have given you command of Applied Thermodynamics (along with other subjects). As shown below You are not the only one who has had additional thoughts as a result of our discussion. It has also made me realise something I should have realised before. Thank you for that. +1 In another recent thread here at SF, a teacher of thermodynamics asked how to introduce the subject of entropy, without using the traditional second law approach. The discussion in your thread made me realise that of course you cannot use much of the mechanism of the second law if you are going to do this. This must be why the early diefintion did not mention entropy : entropy had yet to be defined. Hindsight allows an applied thermodynamicst to use formulae and techniques out of the logical sequence of the definition. This is in fact what I was doing and led me to my original agreement that you cannot use the classical approach to prove or disprove the kinetic interpretation. You need additional material for this. Perhaps my digression to show why the early pioneers always referred to cyclic processes was excessive, but I hope you have come to realise that since the kinetic approach is non cyclic in basis, you cannot use that part of classical thermodynamics which is defined only for cyclic processes. So another way must be found. But the kinetic question in the OP is not applied thermodynamics it is more fundamental than that. So discussion must follow and hold to a formal logical sequence of definitions and results. I don't know if you have heard of the Massieu and Planck functions ? These two provide the (mathematically derivable) link between the classical and the statistical approach, so that this is often referred to as 'the Massieu Bridge'. All of this is expounded detailed in Guggenheim's Advanced thermodynamics. (I would not recommend the Wikipedia pages on this they are rather unhelpful and not completely comprehensive or correct) However, just are there are several approaches to classical thermodynamics, there are several versions of the statistical approach. Unfortunately the statistical versions do not always completely agree with the classical versions, fluctuations being one such area of divergence. Epstein, in his famous textbook, included a whole chapter on the experimental evidence for and theoretical basis of such divergence. Epstein A textbook of Thermodynamics A free pdf is available here. https://archive.org/details/textbookofthermo031032mbp/mode/2up I am not sure of free pdfs for Guggenheim.
-

Spacetime Diagrams Lorentz Transform.
studiot replied to can't_think_of_a_name's topic in Relativity
Taking your equation xB = γ(xA - vtA) = 0 So either γ = 0 or (xA - vtA) = 0 or both. But γ is never zero so (xA - vtA) = 0 Which is not suprising since xA = xB =0 only when tA = tB = 0 substituting (0 - v.0) = 0 So sorry but this line of enquiry goes nowhere. -
Burettes are filled to the zero mark which is at the top of the graduations on the tube. The graduations increase downwards from this zero so indicating the amount run out when the tap is closed and the new level of liquid is read off.
-
Some more examples of zero errors. Mark has a metre rule with a piece of chewing gum stuck on the (zero|) end. This rule always measures too short a distance. Melissa has a different metre rule with the first centimetre broken off. This rule always measures too long a distance. Johannes is slapdash in filling his burette for titrations. Sometimes he overfills slightly, sometimes he does not quite reach the fill mark So his titration readins could be either too high or too low. Wilhelmina often bakes cakes, and weights out the flour into a old ice cream box stood on her digital scale. However she often forgets to zero the scale plus box (this is called the tare function of the scale) so weighs out too little flour by the weight of the box.
-
I agree that the OP definition is not quite right, but both your A and B examples have two errors. Yes a zero error is a systematic error but it is not due to a wrongly marked or non uniform graduation and yes it can result in an increased or decreased actual measurement. A simple example of a zero error would be dirt on the pan of an otherwise accurate weighing scale.
-

Attempting to create a generalized graph of mathematics
studiot replied to ALine's topic in Mathematics
As I think your project is very worthwhile I have been giving some serious consideration to explain my tiling comment. +1 I have been rather busy today but I will post soon on that, using these pillars of Maths Arithmetic, Algebra, Geometry and set Theory in relation to trigonometry and symmetry. Meanwhile perhaps you would like to think about this category ? Mathematical notation and symbols. This is often the Cinderalla category, but it pervades all of Mathematics. -
How many versions of the truth can there be? You are the one who stated plainly that there are zero versions. I simply pointed out that there must be at least one version. This is a non sequitur. I have no idea what you are trying to say here. A particle that is following a specific track, with no opportunity to change its kinetic energy and therefore its 'temperature' and no opportunity to receive or distribute heat is a purely mechanical system. How is that not a constant entropy system ? Since I only drew one single solitary particle and one single solitary track, surely you cannot have mixed them up. You're no nearer to answering the OP paradox now than you were when you first posted at 9.51 pm on Friday. If you don't know then just say so. Frankly, with so many of you stumbling over the 1st Law constraints, there seems little point in discussing the 2nd. Ad hominem instead of Physics yet again.
-
The gas has increased in entropy by W(T2-T1/2)/T1T2 (= W/T1 - W/2T2) Reservoir 1 has decreased in entropy by W/T1 Reservoir 2 has increased in entropy by W/2T2 W/T1 - W/2T2 - W/T1 + W/2T2 = 0 Hence no nett change in entropy. So you have used (confirmed) the Chemists' version of the second law as I suggest for a non cyclic process. Congratulations. Your examples don't come into it. Your assertion (paraphrasing) "it is reversible therefore it is isentropic" is a clearly flawed assumption. Please post accurately the text you claim to be paraphrasing. This must be arrant nonsense. You must have at least one version. No one is trying to 'entrap you' , although you did earlier suggest you had trapped both swansont and myself. Such colourful language is not conducive to cooperative discussion. I asked for your version or statement of the second law and gave the reason that it was to enable us (all) to compare the OP offending process (and any other) with this statement. Why is that trying to trap you or in any way unreasonable?
-
Only if we accept your changing my post to the specific value of work you introduced. If you can only work with that particular figure, rather than the general one I introduced, then we can use your w/2. However that leads to what appears to me to be a self conflicting pair of statements. How can we have "increased its entropy overall by W(T2-T1/2)/T1T2 and also have "there isn't a change in entropy" ? Please explain. ... which I think you need to gracefully withdraw. No shame. Just own the error. I can't see the connection between this and the first example designed specifically to show why the process needs to be cyclic to obey the second law ( as stated by its originators as I have already posted) and the second example which might have been put better, but was designed to show something else. A particle bouncing back and for in a perfectly elastic manner along a predifined track suffers no change in entropy. You have still not stated the version of the second law you wish to employ, despite several requests. That is pretty rude in my opinion.
-
dS = dQrev/T by definition. Your process may be ideally reversible, but it absolutely is not isentropic other than the stage you call 'adiabatic compression'. If you also called this stage 'isentropic compression' it may help alert you to the fact that isothermal compression processes are very far from isentropic. So if you've drawn inferences from this line of thinking that you believe will help me with my box problem (I no longer have one), I fear that you have managed to confuse yourself. Read it again properly and post the extract where I also called this stage isentropic If I did that why do you think I allocated q1,2 to stage 1 - 2 and q3,4 to stage 3 - 4 resulting in a net heat change? So the system entropy changes are [math]\Delta {S_{3,4}} = \frac{{{q_{3,4}}}}{{{T_2}}}[/math] and [math]\Delta {S_{1,2}} = \frac{{{q_{1,2}}}}{{{T_1}}}[/math] What I said was the that net w = 0
-
I have a question about the barchart. I don't know how the state and local funds are raised in your chart. In the UK much of the 'local' spending is provided by central government, only some is raised locally Can you say how this works in other countries? I agree +1 It is a knotty problem.
-
To continue. So how does my process stack up against classical statements ? Well since the process does take transfer heat from a colder body to a warmer one it is a good job that it is not a cyclic process so does not satisfy all the conditions of the classical second law. In other words the Second Law (classical formulation) should not be applied. That example is exactly why the originators included the cyclic requirement. But of course it is unsatisfactory not to be able to apply the Second Law to non cyclic processes, there are many such in Chemistry. This is where the Chemists' version comes in useful. The key to this is now to consider the system and surroundings together as one 'Universe'. This may be applied to my one shot reversible process. The process considering the reordering of particles in a box is also a one shot process. Considering my example the particle track is entirely reversible so there is no change of entropy. However you are wrong to say that the box must be included as part of the system. The system can be anything I want it to be so long as I can draw (define) a boundary round it. Of course an injudicious choice of system and boundary can make calculations difficult or even impossible, as you are finding out with the box. A System is whatever is inside the boundary. The boundary is not part of the system. In the box case I choose the box as the boundary. Thermodynamics provides the exchange variables of work and heat which are not state variables to connect the system to its surrounding across the boundary. I would recommend comparing a thermodynamic discussion of the latent heat of fusion of a pure substance with the multiparticle case for the box.
-
I dunno I think Boris has a better hair do. But interestingly the Chief Pharmacist at a big hospital in th UK will earn more than the Prime Minister.
-
What a pity you are preventing yourself from seeing the answer to your original question, which is contained in the answer to my question. I have never claimed it breaks any laws, (quite the reverse in fact if you read my post properly) or that it will provide a supply of free energy. All I asked was how it fits with the second law (which is what you did).
-
Mrs Thatcher had a BSc in Chemistry. But there is a lot of sound sense in what you say.
-
Wow, what a lot of invective just to dodge answering a question, similar to the one you asked in the OP. The joke is that it is based on one from a textbook entitled Thermodynamics for Chemical Engineers written by three professors from the Dept of Chem Eng at Imperial College.
-
Deja vu ! When I first went to University, I wanted to carry on with Chemistry, Maths and Physics, but Chemistry was my main subject. I was rather ill advised but I did find out that Chemistry was short on the Mathematics side so I tried to 'hedge my bets' by applying for 3 courses in Chemistry and 3 in Chemical Engineering. In those day we had to apply for 6 and finally choose 1 after the school exam results. I went for Chemistry which proved to be a bad decision for me. Subsequently I went back and did Applied Maths , then Civil Engineering then postgrad in Marine Geophysics and Geodesy, also working. So my perspective is unusual. But I symphathise with your dilemma. Yes there are well paid jobs (and poorly paid ones) in Chemistry (especially Pharmacy) but that is also true of Chemical Engineering. But please tell us what part of Chemistry you did well at and found enjoyable at School ? I have always been more interested in Physical and Analytical Chemistry than organic or inorganic or bio Chemistry. That is even though I made a very ham fisted Analytical Chemist.
-
If you don't know how to answer my question, which did not mention a cycle or cycles, then please just say so and don't mess around wasting everyone's time. If there was anything unclear in my description please just ask and I will amplify the point.