Everything posted by studiot
-
What is your attitude towards Driving Anger?---An interesting topic I saw from a research article in an OA journal
Yes I agree that the angry driver is not necessarily the competitive one. But those that are blithely ignorant and unaware of the needs of other road users and therefore not competitive, can be the cause of anger in those others.
-
What is your attitude towards Driving Anger?---An interesting topic I saw from a research article in an OA journal
Not all uncooperative drivers are angry. Consider the situation in a busy city street with continuous streams of traffic in both directions. Now consider the driver who wishes to turn left into a sidestreet thereby necessitating crossing the oncoming traffic. There are many variations of this scenario, but all are expedited by cooperation. Sadly some (many? most?) drivers do not cooperate in this situation. They are not angry, they just don't know any better.
-
Conflation Hypothesis - Minds from all fields of physics welcome
The so called big bang hypothesis is based on four dimensional Relativity. The first Law is an conservation law of a 3 dimensional scalar called energy. This scalar is not conserved in four dimensional Relativity. Also applicable are the conditions of the First Law such that we have to decide if the Universe is an open, closed or isolated system. We do not know the answer to this. So all in all it is just our best guess. Another point, I see you are new and have made 3 posts. You are permitted a total of 5 in your first 24 hours as an successful anti spam measure, you will benefit from later in your membership.
-
What is your attitude towards Driving Anger?---An interesting topic I saw from a research article in an OA journal
Old fashioned London drivers would agree 110% with this first sentence. +1 Responsible for some definitely, but ALL ?
-
What is existence?
Remembering this thread is about existence not actuality nor virtuality nor reality nor any other 'ality. Processes present an interesting aspect of existence. That of time. Finite processes have at least a beginning a middle and an end. Unless these all occur simultaneously so the process is independent of time, the question arises "Can the process ever be said to exist ?" This is because only part of the process 'exists' at any one point in time ie at any present. One small point. Whilst I am flattered that you choose to be so polite to me, I am not female ie I am not a 'Lady'. I am sorry if anything I have said has lead you to believe otherwise. I often say she did this or Jane did that as well as Jack did this etc, in order to promote gender equality in my small way.
-
Worldbuilding for a fantasy novel
It is not necessary to expect your planet / moon system to create this weather on its own. There could be a conjunction with other bodies in its solar system that shaded it or passed it through a dust cloud every 4 years, or near an eccentrically orbiting body or somesuch. Many succesful SF stories have been built on variations of this from The Dragons of Pern to the short stories of The Unorthodox Engineers.
-
What is existence?
The thread is about existence and non existence, not whether that is a physical or something else. Anyone who suggests that holes do not exist should invite me to bring my trusty pin to their next balloon party. As for shadows, has anyone tried growing a peach or an apricot against a North wall in the northern hemisphere? The OP, Alex, has a reputation for week long absences between appearance here (probably has better things to do in reality) so we are all awaiting extra context to be supplied. But at least he has provided a worthwhile discussion thread.
- What is existence?
-
Granite origin mystery.
In all fairness that description is self contradictory. No volcanoes are necessary for the formation of granite. The part about slow cooling is iffy at best since the cooling rate determines the grain size so the actual rock formed does indeed depend upon the cooling rate as noted, but if the composition of the magma is not correct then basaltic group rocks will be formed instead instead of the granitic group. The 'liquid' granitic magma is much more viscous than the basaltic group so tends not flow out in great lava flows but remains underground and cools. Outcropping solid granite is then exposed by weathering/duenudation processes.
-
What is existence?
So you are asserting that processes do not exist in actuality ? Mathematics is a process not a product.
-
Is the description of space-time as "space-time" a bit misleading?
Nothing to do with flipping time, whatever that is. Of course you can work in time units. But that means changinf three axes instead of one So instead of multiplying time by ic, you divide x,y and z by c and measure in seconds.
-
Granite origin mystery.
Starting your speculation with a false premise is unlikely to allow arrival at a true conclusion. https://en.wikipedia.org/wiki/Moon_rock We have no samples from Mercury, Venus, Mars or other Moons or the OOrt.
-
Questions on the Helmholtz machine.
Thanks. +1 Having now looked at the Wiki article, which thankfully was quite short and not embellished with too much jargon that also needs looking up I can say the short answer to your question is yes but there are some serious caveats to observe. From my applied maths side of the issue I would say that this is about what we call mathematical modelling. That is using the known characteristics and responses of some system or structure to predict the characterisitcs and responses of another system or structure which behave similarly (hopefully identically) in some respect. Caveats No model is perfect except the system or subject being modelled itself. The 'match' between system and model normally extends over some range or another. Attempts to model outside this range are very likely to be misleading at best. This is why interpolation, which means bracketing the output between two ( or more) known values, is considered more reliable than extrpolation, which means extending the known range of validity of the model. In relation to the o p question, the Wiki article mentions Boltzman statistical mechanics and the Boltzman entropy formula from statistical mechanics. This formulation rests on the principle of equal probability of all states which does not always hold good. It is a very simplified assumption, which works in many cases but far from all and the exception have lead to much important modern Physics. This important fact is often omitted from descriptions of statistical mechanics. Before I offered you a simple, but perfectly sound, explanation of entropy, that you will not find in most treatments either. This explanation requires about the mathematics available to an 11 year old ie the understanding that area = length time breadth. My offer still stands.
-
What is existence?
I note from all these replies that my original contention that the definition and meaning of existence depends upon context has been borne out. This would further imply that there are multiple definitions of the word (as with many words in the dictionary). This bring me to Alex's second question. Non existence must now be much easier to define, at least for words with multiple meanings, or for complex statements. Here are some examples. A single meaning for the word (insert chosen multple definition word) does not exist. In the recipe for shepherd's pie, beef mince does not exist. Ain't complexity wunderful ?
-
Questions on the Helmholtz machine.
Good morning. I, for one, have never heard of the Helmholtz machine so I don't doubt your information. However a reference would be helpful so that we can learn something more about it. Thank you.
-
A mass can be be lifted with force less than its weight
So why does it go down ?
-
A mass can be be lifted with force less than its weight
I don't believe I said any of this. Clearly the countermomentum must act over the same timespan so nothing is moving 60 times faster. But the scale spring or whatever must have stiffness so that the movement of its scale over that same timespan is only some fraction of the moving mass of the body. I'm also not saying that when straightening up the bulk of the body mass moves anything like 1 metre. If you are refering to the video it involved starting flatfooted on the scale and raising the body slowly to tiptoe, about 0.1m or 1/10 of that figure. I also asked for some help posting a short video of the scale acting as I described. Photographs I can do but they are not useful in this case.
-
Effects of a (liquid) water canopy
Are you another Science Fiction Author looking for help ? Quite a few have been helped here. Or are you referring to Genesis 1:2 ? Or what is your purpose ?
-
What is existence?
+1 I said it is complicated. Thankfully the English language has (nearly) the wherewithall to deal with the subject. I have already pointed out the connection between time and existence.
-
A mass can be be lifted with force less than its weight
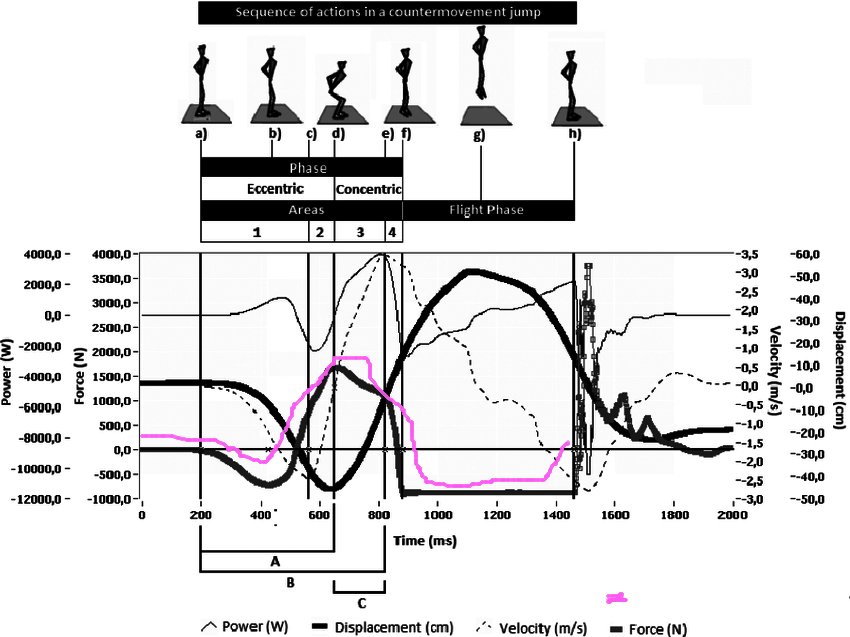
I found this article and measurement of the reaction force from the ground on a person performing a standing jump. https://www.researchgate.net/figure/Dynamic-and-kinematic-curves-for-the-countermovement-jump-sample-output_fig1_233734813 It includes this force plot I have highlighted in pink. Note the researchers recorded a dip, as I did. I agree, Newton rules OK. How about this. You stand on the scale You are quite still. So the vertical momentum is zero. You lunge upwards, acquiring vertical momentum. Then come to a halt and are again still. Before and after the vertical momentum is zero. During the upward lunge this must still be true ie there must be a counterbalancing downward momentum somewhere. In the scale mechanism. So the mechanism reads a lowering of weight. The faster the upward lunger the greater the rate of change of vertical momentum and the the greater the corresponding reduction in scale reading. I am far from an expert in human body mechanics so I would welcome comments from those who are as to how this might work.
-
What is existence?
I wish it was as simple as you seem to think. First off 'does' is the present tense of a verb. Are you including other tenses Will my dinner exist tomorrow ? Did the dinosaurs exist ? Then the there is the subject, which is a noun. English nounds can be concrete,( like your apple) or abstract Does Harry Potter exist ? Does my reflection in a mirror 'exist' ? Does the centre of a torus (donut) exist ? So context please ?
-
What is existence?
I think you need to provide substantially more context to engender a sensible discussion.
-
Space charge density
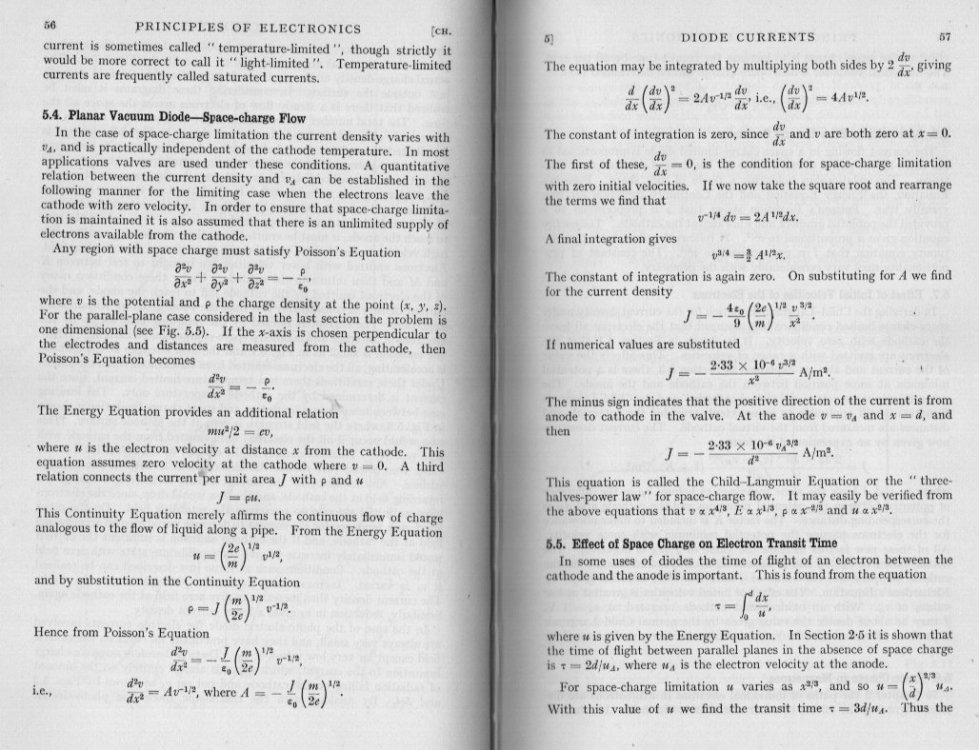
No it does not mean that at all. You 40 page document derives analytical conditions for a simplified model in one dimension. Note this analysis is compatible with my attachment which uses Poisson's equation for the flow. The simplest model is Laplace's equation [math]{\nabla ^2}\left( \Phi \right) = 0[/math] This does not account for interaction between paticles so for a stream must be replaced by Poisson'r equations [math]{\nabla ^2}\left( \Phi \right) = f\left( \Phi \right)[/math] Where phi is a suitable flow variable. Do I understand you are interested in the the method outlined in your eference document here is the summary whihc would have been useful for you to post I have emboldended the important descriptive sentence.
-
What is gravity
I'm guessing you are studying high school physics or applied maths, and what you are referring to is the vertical acceleration due to gravity felt by all material bodies on Earth. This is normally given the symbol g and has an (average) metric value of 9.81 metres per second per second, you you are correct this is an acceleration.
-
Space charge density
Gosh it's a long time since I looked at this subject. I think some more information is required. What do you mean by flowing gases ? The electron ballistics of vacuum or near vacuum devices and those which have an appreciable gas fill are quite different. Also relevant is the question of what frequency are you working at D.C. or (ie zero) or some A.C. frequency. You have mentioned space charge, which normally refers to a particular effect in such tubes, not the charge density of the flowing current. Please confirm what you actually mean. I'm guessing but are you referring to this ?