-
Posts
18435 -
Joined
-
Last visited
-
Days Won
107
Everything posted by studiot
-

Explained - What is Scientific Chaos Theory?
studiot replied to Science & Universe's topic in Physics
Very wise. Meanwhile this is a science discussion site, so perhaps you might like to offer some specific Science ? Chaos theory has now developed to the point of covering a wide range of effects and phenomena and a substantial depth of Mathematical Theory. This theory is quite separate from probability theory, perhaps you wish to explore the link between the two ? -
I didn't ask if the project was worthwhile or even to whom it might be worthwhile, though yes I made my opinion of it clear. I asked if the social changes brought about by Covid make it redundant. Please discuss the question asked.
-
Interesting topic to raise. +1 Just a small question for a Tuesday huh ? I will do some investigation, hopefully others with information might chip in here. Meanwhile can you say if the concrete being poured was foaming or aerated (lightweight) concrete? That is what I would recommend for filling (scour) voids in soft ground like there is there. I have done this on the Somerset levels. I seem to remember a discussion thread about the Mississippi before. Was that yours also?
-
Your question will become much more understandable when you set out all the details. The biggest question at the moment is : Why is there an eddy ? Eddies do not just appear, something causes them.
-
Government after government has committed increasing and enormous sums to the HS2 rail project so that a handful of businessmen can travel from London between Birmingham a few minutes more quickly. Apparantly they claim this is essential to the economic wellbeing of the UK, some even suggest the country would collapse economically without it. Along comes Covid and what do you know? Businessmen suddenly do not need to travel betwen London and Birnmingham. The age of videoworking has arrived. My question for discussion lies in the title to this thread.
-
Well I'm glad you got your answer. However I'm disappointed with your response to my earlier question, which I asked for a particular reason, I had intended to develop for your benefit. In my first encounter with cosines I was given this definition, which referred to right angled triangles only. Pythagoras had already been proven. \cos \psi = \frac{{adjacent}}{{hypotenuse}} Using this definition can you see that there is a problem with this, in respect of your question ? Since c has to be the hypotenuse, which of the two sides corresponds to the adjacent side ? a or b ? Clearly there are two possible answers here and the solution to this problem is geometrically interesting. It is very important to get this straight in your head because both Pythagoras and the Cosine rule are deeply embedded in fundamental Physics.
-
Did you really learn about the cosine rule before you learned a definition of the cosine? What is your definition of a cosine?
-
Who is John ? Was he a baptist? I agree That’s not a full answer. Is “optimum for plant growth“ the goal? Why should that be? None of us here actively participating (including by video) are paleobiologists or paleoclimatologists. Perhaps we should all read what they have to say ? Carbon dioxide is (apparantly) not a limiting a factor. Plants did not delvelop from the oceans and land margins for half a billion years. When they did, plants had no leaves. In fact plant development did not blossom until plants developed solar panels (leaves). This was followed by a rapid development of plants in a period of declining carbond dioxide levels. Again apparantly the whole process is controlled firstly by the KNOX gene and subsequently by the HIC gene. (MY note there is probably more to it than this) Reference The Emerald Planet David Beerling.
-

I have a new theory about the origin of earth.
studiot replied to farsideofourmoon's topic in Speculations
This shows lack of proper consideration. How I wish folks would give some proper consideration before they posted rather than just looking in the mirror after one too many pina coladas. Very clearly if the Earth was captured from somewhere else then it was formed somewhere else, before it was (could be ) captured. So this thread is not about the origin of the Earth but about a conjectured later event in its history. Please think before you post. -
Go is a fixed value, not a (state or otherwise) variable. As such its derivatives are zero, unlike G which is a variable. When I was preparing my last post i thought I found Matthew both asking for the variation of enthalpy with temperature and saying that it did not vary or that the variarion was negilgable. I was going to reply to that but I can't find it again so I must have been mistaken. (Sorry in advance Matt) Three book recommendations and a gripe In 1925 P W Bridgman published "A condensed Collection of Thermodynamic Formulas" - Harvard University Press Where he tried to provide comprehensive lists of thermodynamic variables and the first and second derivatives, in terms of each other. Quite an undertaking. In 1949 E A Guggenheim published Thermodynamics, an Advanced Treatment for Chemists and Physicists. - Horth-Holland Publishing This has been used as a reference bible in many later 'new wave' Thermo books In 1994 G Carrington published Basic Thermodynamics Oxford Science Publications I consider myself lucky to have a copy of this since a quick net check shows it is in very short supply, but great demand, all around the World. It really is a first class modern book for all those who need thermo science - Basic is a misnomer here but his explanations and treament of the complicated parts couldn't be simpler or clearer, all the way up to sub atomic thermo spin systems. Carrington is one of the select band of geniuses produced by our Southern Cousins in Aus and NZ. They seem to possess a special clarity of thought. Oxford have two companion volumes Binney Theory of Critical Phenomena Yeomans Statistical Mechanics of Phase Transitions. So my gripe. It seems to me that everyone who writes about Thermo is dissatisfied with the notation and invents his or her own which is claimed to be better, but really adds to the confusion. I include Proff Atkins here. Free Energy is the hilarious part. Gibbs was American. Helmholtz was German. So the Americans use the symbol F for Helmholtz Free Energy and the Germans use the symbol A (Which I have done as well)
-
I think that buried in all that maths are some conflicting assumptions so let me offer you an alternative. The Gibbs-Helmhotz equation is not one equation - it is a bunch of equations and part of the trick is to pick the right one for your purposes. Usually this involves using a form that converts a quantity that you know or can easily determine by measurement into a quantity that you don't know but want to. You also need to use a form that offers an alternative extensive (intensive) variable to substitute for your unknown extensive (intensive) variable. You need both extensive and intensive variables to multiply together to get the units of work/energy. So here is one form of GH connecting the free energy of a reaction to the heat of reaction and temperature. \Delta H = \Delta G - T{\left( {\frac{{\partial \Delta G}}{{\partial T}}} \right)_P}...........1 This is easy to derive, are you happy with that? In state variable form this can also be written without the difference operator delta which gives the ex tensive quantity as a difference (products - reactants). H = G - T{\left( {\frac{{\partial G}}{{\partial T}}} \right)_P}..................2 here are a few more that I will not use for this question, but may be useful elsewhere. U = A - T{\left( {\frac{{\partial A}}{{\partial T}}} \right)_V} U = H - P{\left( {\frac{{\partial H}}{{\partial T}}} \right)_S} H = G - T{\left( {\frac{{\partial G}}{{\partial T}}} \right)_p} A = G - P{\left( {\frac{{\partial G}}{{\partial P}}} \right)_T} G = A - V{\left( {\frac{{\partial A}}{{\partial P}}} \right)_T} Returning to equation 1 dividing through by T2 and rearranging we have \frac{1}{T}{\left( {\frac{{\partial \Delta G}}{{\partial T}}} \right)_P} - \frac{{\Delta G}}{{{T^2}}} = - \frac{{\Delta H}}{{{T^2}}}................3 Now consider the function \frac{{\Delta G}}{T} Differentiate with respect to temperature \frac{\partial }{{\partial T}}\left( {\frac{{\Delta G}}{T}} \right) = \frac{{T\left( {\frac{\partial }{{\partial T}}\Delta G} \right) - \Delta G.1}}{{{T^2}}} = \frac{1}{T}\left( {\frac{{\partial \Delta G}}{{\partial T}}} \right) - \frac{{\Delta G}}{{{T^2}}} By the rule for differentiation of a quotient. But this is the left hand side of equation 3 Thus \frac{\partial }{{\partial T}}\left( {\frac{{\Delta G}}{T}} \right) = - \frac{{\Delta H}}{{{T^2}}}......................4 Integrating, and introducing an arbitrary function of P since this is a partial differential equation \frac{{\Delta G}}{T} = - \int {\frac{{\Delta H}}{{{T^2}}}dT + L(P)} ..............5 Alternatively we may avoid L(P) by integrating from a known condition T1 to some T2. \frac{{\Delta {G_2}}}{{{T_2}}} - \frac{{\Delta {G_2}}}{{{T_1}}} = - \int_{{T_1}}^{{T_2}} {\frac{{\Delta H}}{{{T^2}}}dT..............6} Now we need the enthalpy as a function of temperature. assume a converging power series of the with base Ho and coefficients alpha, beta etc \Delta H = \Delta {H_0} + \alpha T + \beta {T^2} + ..... + ........7 This yields the difference in free energy as a function of temperature \frac{{\Delta {G_2}}}{{{T_2}}} - \frac{{\Delta {G_1}}}{{{T_1}}} = \Delta {H_0}\left( {\frac{1}{{{T_2}}} - \frac{1}{{{T_1}}}} \right) - \alpha \ln \left( {\frac{{{T_2}}}{{{T_1}}}} \right) - \beta \left( {{T_2} - {T_1}} \right)......8 If you prefer a direct formula then you must address the issue of L(P) \Delta G = \Delta {H_0} + L\left( P \right)T - \alpha \ln (T) - \beta {T^2}...........9 This isn't so bad as it will be a constant at any given pressure. But is does mean you have to obtain a value of delta S or delta G at some particular temperature.
-
Thank you for posting more detail. The material is still there, much of it in the same words in all these editions, except that in my 5th ed (which I think is the best one) there is a warning additional to the excerpt you posted. "Note that in the definition of ΔrG0 , the Δr has the normal meaning as the difference products - reactants." The two pages I posted hold what I think is the key to your dilemma. If you look at the middle of the second page you will see the warning "We must be careful to distinguish the reaction Gibbs energy, the slope of G at a specified composition, from the standard molar reaction Gibbs energy ΔrG0 In this case Δr has its normal meaning of the difference of two quantities." Reading both the two pages I posted explain that one of two similar sounding quantities is a a slope of a graph (of G v an extent of reaction parameter), the other is a difference in values of G itself. As a matter of interest I cannot get Latex to offer the london underground (strikethrough zero) symbol but +1 to Scienceforums as I can manufacture it with the superscript and strikethrough tools on the site editor.
-
The G-H eqaution is derived for conditions of constant temperature and an infinitesimal change of temperature. I don't see anything like yours in either my 5th or 6th Atkins. The relationship to enthalpy comes in as the work term w' Gibbs-Helmholtz (original equation) \Delta H + w' = T{\left( {\frac{{\partial w'}}{{\partial T}}} \right)_P} but w' = - \Delta G So \Delta H - \Delta G = - T{\left( {\frac{{\Delta G}}{{\partial T}}} \right)_P} Is the form of the equation in Atkins.
-
Sadly I see I have pasted in the same equation twice. Hhere is the correct one for many electroned atoms. H = - \frac{{{\hbar ^2}}}{{2m}}\sum\limits_i {\nabla _i^2} - \sum\limits_i {Z\frac{{{e^2}}}{{{r_i}}}} + \sum\limits_{i > j} {\frac{{{e^2}}}{{{r_{ij}}}}} My apologies to all.
-
-
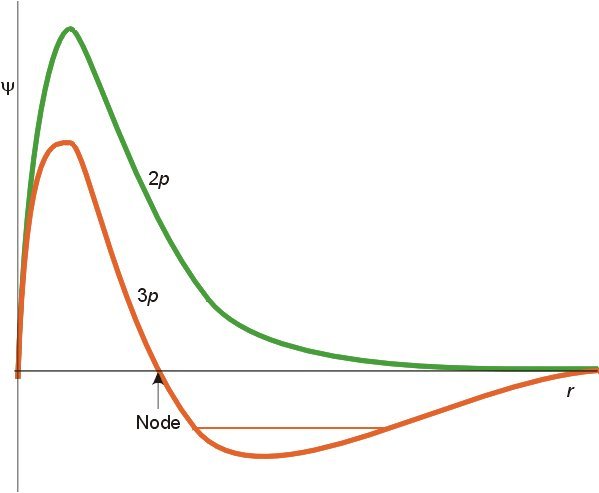
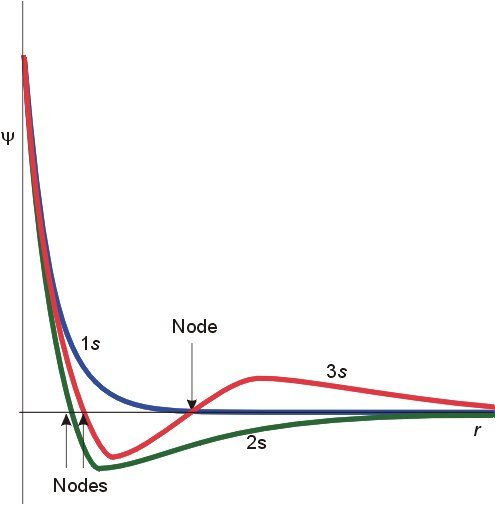
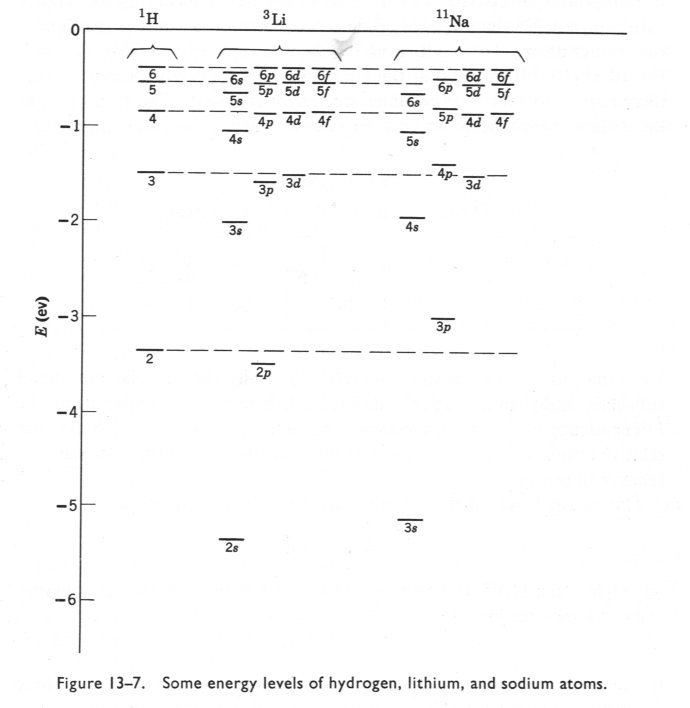
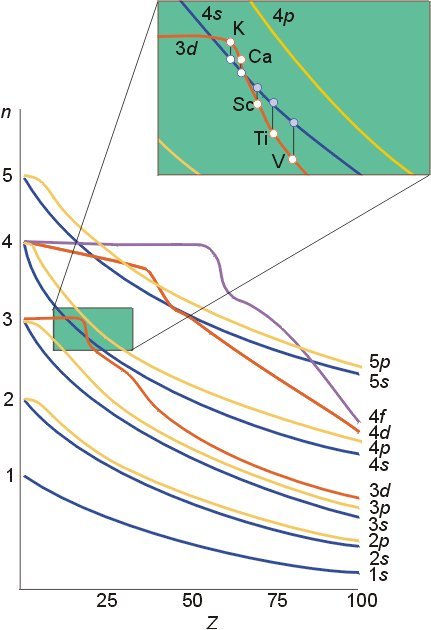
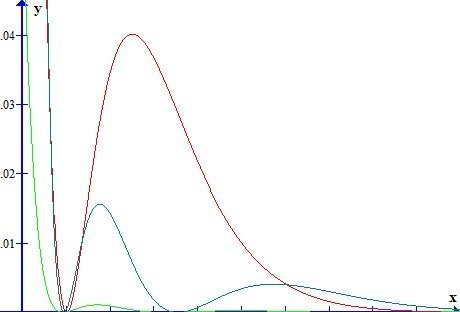
I said it would take a while to answer but first a word of gentle friendly advice. This must be a very small part of your first year curriculum. I have never heard of Chemical Engineers requiring such detail. So don't be diverted from the main thrust of your subject, there is so much introduced in the first year, from fluid mechanics to science of materials to advanced mathematics to thermodynamics to transport phenomena...... Remember that this is only about atoms. So think about how few times a CE will deal with pure atoms as opposed to the great many times he will deal with molecules? So to proceed with atoms. I will try to clear up a few things you may know or may only partly know on the way. Firstly the Hamiltonian in a system (H) relates the total energy of a system to some controlling parameter. In the case of wave mechanics the Schrodinger equation this is the wave function. We say that the Hamiltonian operates on the wave fucntion to ouput the total energy. H\left( \psi \right) = {E_\psi } Now this can be broken down to measurable quantities such as momentum, charge, mass etc For a single electron atom Ie a hydrogen-like atom this becomes H = - \frac{{{\hbar ^2}}}{{2\mu }}{\nabla ^2} - Z\frac{{{e^2}}}{r} Where Z is the atomic number that is the number of protons in the nucleus. So the energy depends upon the number of protons in the nucleus. Further this equation has been solved analytically. Some notes here are in order. A hydrogen-like atom has one outer electron over a core of fully paired, if any, electrons so includes lithium, sodium, potassium etc. This electron will inhabit an S orbital, generally of higher energy than any orbital in the core. More of this later. Your diagram refers to S orbitals only, and further more is proportional to the square of slutions of the Schrodinger equation. Since it is a squared term it is non negative ie positve or zero. Plots of the wavefunctions themselves show positive and negative regions. The various humps in the square (probability plot can be seen forming in these plots) Further the S orbital is the only one that is not zero at the origin (I have only show p but this is also true of d and f orbitals). The second point is as already made; the actual energy value depends upon Z. So the actual energies of 'the same' orbital in the atom of one element will be different from those in the atoms of other hydrogen-like atoms. Note carefully on this chart Such variation of levels becomes even more complicated when we consider atoms with two or more outer electrons Looking back at the Hamiltonian this is because we need to add more terms due to the interaction of the other (Z-1) electrons. H = - \frac{{{\hbar ^2}}}{{2\mu }}{\nabla ^2} - Z\frac{{{e^2}}}{r} Now the electrostatic energy is increased by the addition of this interaction energy. Unfortunately even this simple addition cannot be solved analytically. This accounts for the majority of the effects and the reason why all the building up , aufbau, Hund, Slater diagram, Mollier etc rules have exceptions. And all this effort is only for less than 0.01% of the substances a CE will be dealing with. For molecules and chemical bonding several more terms involving cross products of the Z numbers for the second and subsequent nuclei in the molecule are needed. This is why the build up of atomic orbitals, which was greatly studied in the first half of the 20th century, faded in the second half, when the subsidiary Science of Computational Chemistry was born. https://en.wikipedia.org/wiki/Computational_chemistry How are we doing?
-
-

Confused About the Earth's Early Atmosphere...
studiot replied to jimmydasaint's topic in Earth Science
Thank you for this I didn't know it. Biology is not my strong suit. +1 I did find an interesting full description of the chemisty of photosythesis (so far as it is known) inProfessro Downes' book The Chemistry of Living Cells. Apparently tracing the carbon with C14 identifies something like 11 stages in the order in which the chemicals must form. -
Sorry if my answer was too basic and simple. A detailed answer to both this question and your Moellier question will take some time to answer. But do not expect formulae they can only give trends. Solution of Schrodinger for the exceptional cases can only be done by numerical methods which involve a best guess and refining this to fit observed data. Meanwhile read this recent thread about exceptions.
-

Confused About the Earth's Early Atmosphere...
studiot replied to jimmydasaint's topic in Earth Science
I don't know any married verbs, do you? -

Matter that gets hard when cold water is applied
studiot replied to lucaspost's topic in Organic Chemistry
Yup more care needed +1 -
Thank you for the change of grammar, but changing the grammar, as well as the tense and the mood entirely misses my point. Further it makes a nonsense of this statement of yours, since it fails to progress to the future.
-
Not sure why you need this for Chemical Engineering but good on you for wanting to know. As a ChemE student you will be familiar with differential equations in general and the separation of variables in particular. The good news is that because the potential energy of an electron in an atom is a function of distance from the nucleus only it is possible to separate the variables in the quantum differential wave equation (Schrodinger etc). This means that we can express the wavefunction as the product of three functions, each one only involving one of the three coordinates So we have in spherical coordinates \psi = R\left( e \right)\Theta \left( \theta \right)\Phi \left( \phi \right) So of the four quantum numbers, n, l, m and s m is the magnetic quantum number and s the spin number and do not contribute to potential energy. For an S shell electron l = 0 and the angular part is therefore constant. So we are left with the radial part R(r) The probability of finding an S electron in a small element of volume, dv at a distance between r and (r + dr) is then [R(r)]2dv. Also the probability of finding the electron anywhere between between r and (r+dr) That is lying in a spherical shell of radius r and thickeness dr is given by replacing dv by the volume of the shell volume = 4πr2dr So we have The probability = 4π[R(r)]2 r2dr and the function 4π[R(r)]2 r2 is the radial probability distribution function you have plotted. Does this help?
-

Can anyone tell me about light-resistant materials?
studiot replied to AmethystFloris's topic in Classical Physics
Vitamin B12 injections sometimes come in dark brown ampoules in a box but the daily injection regime usually only lasts a week then tails of quickly to three monthly intervals. So keep them in the box until you want to use them, remove only one at a time. You could wrap the box in foil, rather than the ampoules if you really must but I rather doubt the manufacturer would offer something unsuitable. Keep the box in a cool/cold dark place such as a refrigerator. Storage If you need to store Neo-B12® Injection, keep it in the original pack until it is time for it to be given. ... Keep Neo-B12® Injection in a cool dry place, where the temperature stays below 25°C. Do not store this medicine or any other medicine in the bathroom or near a sink. ... Keep it where children cannot reach it. More items... Neo-B12 Injection - NPS MedicineWise -
I suggest the problem here is that energy and momenta (in the plural) have to do with determinism, which was introduced many pages back. This is because both in classical and quantum theory, knowledge of all the momenta of a system at any instant will entirely 'determine' its subsequent trajectory in phase space. Note momenta has a special meaning in this case which includes the normal mass x velcoity type. Two things about this. Firstly I think the relationship between 'determinism' or fully determined and a scale of indeterminism is similar to the relationship between 'certainty' and a scale of uncertainty or probability. I often have to remind folks that a probability of 1 has more than one meaning. Secondly there is the question introducing of Chaos theory to the mix. Returning to free will Go back to my example of ice cream flavours. But this time you have a choice of vanilla or chocolate and you are only going to have one ice cream. A priori you have a choice and therefore can exercise the high level 'free will' as offered by Eise. But this changes, at the instant of choice, since you can no longer choose say vanilla, having ordered the chocolate. So a posteriori you do not have this free will. This is rather like the famous Monty hall problem, which alters the probability of things between a priori and a posteriori.