-
Posts
18314 -
Joined
-
Last visited
-
Days Won
104
Content Type
Profiles
Forums
Events
Everything posted by studiot
-
Elementary quantum teaching has a ladder of energy levels and asserts that only pohotons with an energy exactly equal to teh energy difference between two levels are allowed. Thus mononchromatic light or line spectra are predicted. In reality the lines are observed to have a non zero width. The radiation emitted or absorbed is only monochrmatic to the extent that the molecular energy levels themselves are sharply defined. If the energy gap has an uncertainty Δε then applying the Uncertainy Principle yields that the frequency of the photon has uncertainty approximately equal to Δε/h. Experimentally this appears as a line half width (Δν) at half the maximum intensity. The time associated with this uncertainty is (2πΔν)-1. Spectroscopists usually consider this as the time over which the emission or absorbtion takes place. What about my request for your definition of emergence?
-

Boltzmann Brains (split from Spacetime is doomed.)
studiot replied to Conjurer's topic in Modern and Theoretical Physics
Gosh wriggle, wriggle wriggle. Why is that ? Why not a straightforwrd yes or no to a straightforward question? Here is my straightforward answer. Boltzmann does indeed refer to states. That is how he derives the probability. BUT There are no states defined in a turbulent system. Because the Boltzmann equation describes the distribution of some state variable of interest over the totality of states available to any system this distribution can be used as an exact calculation of probabilities of any given state. But state variables are only defined for defined states, which are of course equilibrium states. (So yes this comes from Thermodynamics) But the equation offers no information about that state variable when the system is not in a defined state or how it passes through any region to get from one defined state to another. On the other hand since there are no equations that can exactly predict variables in a turbulent system because it is not in equilibrium, we are left with empirical measurements. Some of these are probabilities, since the results vary over a range, and some are pretty tight, for example the fanning friction factor in a pipe. That is the difference. Boltzmann is the result of theoretical calculation. Turbulence is the result of empirical measurement and observation. Of course once we have measured empirically we can compare with known distributions and say Well hey there, it looks a bit like Boltzmann, if considered locally. So for want of something better perhaps we can use Boltzmann as a model for small enough spaces. -

Boltzmann Brains (split from Spacetime is doomed.)
studiot replied to Conjurer's topic in Modern and Theoretical Physics
That is not an answer. Are you stating that Boltzman considers a state or states or are you referring to turbulence ? -

Boltzmann Brains (split from Spacetime is doomed.)
studiot replied to Conjurer's topic in Modern and Theoretical Physics
What considers an average state? -

Boltzmann Brains (split from Spacetime is doomed.)
studiot replied to Conjurer's topic in Modern and Theoretical Physics
I sorry I have no idea what you mean. My point was put as a simple question which you have not even attempted to answer. -
But not replying to my comments on emergence. The least you could do would be to define the term as you see it. In connection with not replying, you totally ignored my main point about spectroscopy. If you did not know this you could have asked for more information. If you did already know this your statement it was set against is disingenuous since it refutes your statement completely. Has he succeeded?
-
Actually it does under the Uncertainty principle. The emission time of a photon can be related to the line width of the spectral line produced (or the other way round) This is very important in spectroscopy as it controls the resolution available. I would suggest that much of your difficulty arises from your attempts to introduce QM into Relativity. I do not know of any successful attempts in that direction. Introducing relativity into QM has been managed, for instance the relativistic Schrodinger equation was derived by Dirac in the 1930s. But marrying the two is still a long way off.
-
And what is wrong with pure maths? (although this is applied) I'm sure with you are happy with the idea of 3 apples. But you couldn't go down to the greengrocers and but a 3. 3 is pure maths. You could buy 1lb of apples but could you buy 1lb ? You would no doubt be happy if I showed you a temperature distribution plot of the temperature in a pot of heated water. But you couldn't grab the temperature from one point and stick it somewhere else. Does this help? Apologies to both of you. Not a problem. Good joke actually.
-
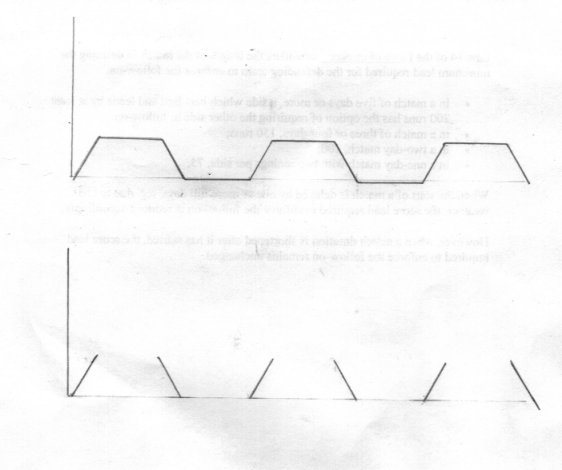
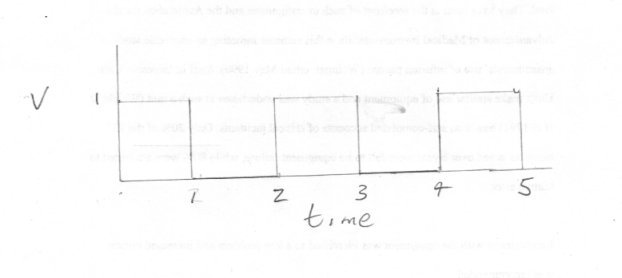
@swansont scuddyx promised to answer me and has kept his word so please let the thread run at least a little longer. First let me say thank you for continuing the conversation and explain tha V represents the quantity etc under scrutiny for change. I know I labelled a time axis but I would like to consider the implications of not having a formal axis at all and then show that we can't do without it. This is quite different from an emergent phenomenon (as I understand the term) which arises as a result of specific and particular circumstances with a particular collection of objects. It cannot be linked to other phenoma taking place elsewhere (ie is independent of them). A good example is the action of an arch bridge. The massive strength of an arch bridge only occurs with a particular configuration of the arch stones and only when they are all there. The arch will not stand up, let alone support load, untill all parts are in place. If one is removed or missing the arch will collapse. A variation on this which shows that the remarkable emergent phenomenon is the fact that the shape causes the apllied force to be diverted through exactly one right angle is the ring. Load a part ring radially and it will bend. Apply that same load to a complete ring and the radial force will be turned into a circumferential one. To return to my diagrams. Say we repeatedly sample the variable under scrutiny, V. When change occurs we expect continuity in V. That is there is always some value for V (including zero) and there is never a sample for which there is no value. There are no gaps in the continuum, and every value is distinct and different so V1 is different from V2 is different from V3 (although it may be the same as V1) Now my plots show how we can distinguish V1, V2 and V3 using the second property I mentioned, that of order. If V1 = V3 but not V2 we have two changes using the order V1 V2 V3 but only one change using the order V1 V3 V2. So our running variable distinguished this difference. Consider a 50kg lump of Uranium slowly degrading through fission. V1 = 50kg V2 = slightly less than 50 kg because of radioactive decay. V3 = slighly less again. and so on But if all this decay occured together as one big change, not as a lot of small ones we would have a nuclear explosion instead of a slowly degrading lump.
-
The quantum wave function does not, in general, oscillate. It has the units of metres-0.5 and exists in a function space that is the Hilbert space of square integrable functions (in a state space of 1,2 or 3 dimensions) Edit In fairness the units I mentioned refer to the one dimensional case. The wave function's units change according to how many dimensions you are working in as it is the square root of a number divided by either a length or an area or a volume. So it is really metre-0.5 or metres-1 or metres-1.5 depending on this. This is unlike the quantity mass which does not change units with number of dimensions (though density and volume do) The square integrable part allows the mathematics to form (and solve) the line, area or volume integrals [math]\int {\Psi {\Psi ^*}} dx[/math] [math]\int {\Psi {\Psi ^*}} dA[/math] [math]\int {\Psi {\Psi ^*}} dV[/math] as appropriate.
-

Does hydrogen have a “radiant state” and a “steady state”?
studiot replied to chemguy's topic in Speculations
Two ?? -

Light does not "travel" (split from Travelling light...)
studiot replied to Dr.Krumpet's topic in Physics
I think the original question about Jedi like light beams has been well answered. But the issue of propagation has been less well explained. It is easiest to talk of light as a wave for this since we can then talk of a wavefront. (It is also possible to ignore the dual nature of light) When light is emitted it spreads out as a (wave) front. That front travels at the local 'speed of light' in th medium of transmission (which may be a total void). The entire space between the emitter and the front and the emitter (ie behind the front) contains light (ie is illuminated) until the emitter stops emitting. When the emitter ceases darkness spreads at the same rate at the wavefront, as does any change to the light intensity. -
Welcome Johnny. What you need is a high heat capacity material that won't boil or burn so a metal flask ofwater is out. A brick or concrete block springs to mind as does a piece of cast iron. You also say that weight is a consideration, but don't say whether you want more or less weight.
-
From EU public document https://ec.europa.eu/jrc/en/health-knowledge-gateway/promotion-prevention/nutrition/sugars-sweeteners
-
I'd be interested to hear how he explains how an electromagent picks up a scrap car in a scrapyard ?
-
But the maths in my diagrams is very simple. Further you should be able to explain stationary waves, which do not change with time, but are simple but essential parts of quantisation.
-
Then you will be able to domonstrate your claims mathematically please. Why do you bother to ask when you don't reply to them?
-
Hello Eise, you seem to have missed my response(s) in our conversation.
-
I hadn't looked before but I see this is posted in quantum theory, so is this comment conditioned by that ? I find the discussion itself interesting (even if it might be in the wrong place). But I don't think change is the right word. Time is an independent variable that we use to measure difference, congruence and similarity. There does not have to be a change for these to occur. Edit this also fits with Strange's cross posting.
-
This does not address my comment about the order of the changes in a sequence. So both continuity and causality are stymied.
-
Because there are changes between them. This is why I numbered the changes, if you like you could make each change different so that they have distinguishing characteristics. That might be an improvement on my model since I have made them of only two types.
-
Thank you MigL Actually Huck has done us a service here since my second diagram illustrates this issue much more forcefully. If we apply the definition that time does not exist in the flat sections of my first graph time becomes a sequence of disjoint points along the axis, with large gaps between. So how do we distinguish the points where time exists I have labelled 1,2,3,4 etc ? How do we preserve the order of these points if there is nothing in between them so they are piled up one on top of the other? Moving on to the second (pun intended) diagram, we now have a sequence of isolated slashes, illustrating the same issues. So this implies that time is discontinuous. Does this tally with our experience? So sorry to disagree with you Eise, but there must be some things we can deduce about the gaps.
-
So have I seen these phenomena. But what does that have to do with the OP and my response to it? If it makes you more comfortable, you can consider a trapezoidal shape but this part (the important bit) still stands.
-
I really don't understand this. I omitted the diagram from my post so I went back in plenty of time and uploaded it as an edit. I checked that the diagram was in place, but now it is gone. This should help make sense of my post.