-
Posts
18311 -
Joined
-
Last visited
-
Days Won
104
Content Type
Profiles
Forums
Events
Everything posted by studiot
-

Linear Modelling In Systems Biology
studiot replied to HenriSpoor's topic in Biochemistry and Molecular Biology
There is some discussion of this subject, plus a few examples to test your algothirms on in Elementary Numerical Analysis Conte and de Boor Page 398ff Numerical methods for Differential Equations Ortega and Poole Page 84 to 118 But I have not come across any biological applications. -
It's sometimes difficult to draw the line between trying out new ideas and flights of fancy.
-

Variations and consequences of the Laws of Thermodynamics
studiot replied to studiot's topic in Engineering
We are having a good conversation so take your time as needed. I look forward to your response. Just be aware that you have made difficulties for yourself by demanding an isolated system. There can be entropy changes, but not in an isolated one. As to Tex or MathML You can use the greek characters available on your computer within the fonts. In windows run Charmap.exe and copy the required character directly into the typing. I don't know hoqw to access the fonts in linux, but they are still there. Alternatively free wisywig maths editors that allow you to build up a maths expression for direct copy and pasting into the forum are available at https://www.codecogs.com/latex/eqneditor.php or http://www.sciweavers.org/free-online-latex-equation-editor -
No apology needed. Beguyer (1862) and Newlands (1864) published idea of aranging the then known 50 or so elements in order of atomic weight, based on Hydrogen = 1 and Newlands floated the idea that heavier elements were built up from Hydrogen. This was ridiculed by chemistry peers as being no better than arranging them in "alphabetical order". Meanwhile Meyer published a 'periodic' grouping of these lists in the second edition (1970) (1870) of his book, and (My thanks to the member who pointed my slip out) Mendeleyev made the bold leap by swapping iodine and tellurium in the list, making it a table not a list, and leaving spaces for three elements that were discovered in short order as a result, published in 1869.
-
It's good to bear that in mind.
-
Yes I see what you are saying, but this could also be interpreted as meaning that Science cannot progress because you should never consider anything the Science 'does not support' and so will never test it. The history of the periodic table provides a fine case in point.
-
From what I can see you are running smack into the difference between the way Mathematics and Physics treats graphs in general and multiaxial graphs in particular. In Mathematics scales along (all) the axes are just numbers. Physics adds units to these numbers to give them meaning. So either you can say that physics allows you to endow meaning that is not inherent (there) in the Mathematics, Or you can say that in Mathematics you can do things you can't do in Physics.
-

Variations and consequences of the Laws of Thermodynamics
studiot replied to studiot's topic in Engineering
What's tricky is knowing which equation to use when and how this relates to the system definition. Each way brings its own challenges to overcome. Engineers have one way to do this, based on open systems and a control volume. You can also obtain your expression of entropy change using statistical mechanics. -
What a lovely piece of Philosophy. +1 I think the best Science has to offer on thw question is the mathematical idea of the universal set, This concurs with english language usage as well, meaning colloquially "everything to do with the job in hand".
-
To answer this one would need to know what exactly you mean by random (adjective) and randomness (noun) and probability. Probability is a comparative measure, given by a number, of a particular result of a process, compared to other possible results. Random is an adjective applied to that process itself, although we also bestow the title 'random result' on the result if it is deemed to have been generated by a random process. So the Kolomgorov definition of a random number is one arrived at by the the explicit statement of the number as being the shortest process of generation.
-

Variations and consequences of the Laws of Thermodynamics
studiot replied to studiot's topic in Engineering
Here you have a problem with the initial conditions, if you conside the system to include the second chamber (B). Let us look at the description of the 'system' more closely. Scenario (1) - Your description (please correct this if I misunderstood) The system comprises the contents of chambers A and B. The system boundary is formed with isolating adiabatic impenetrable walls. But thus boundary must sorround both chambers A and B at all times. However this system is not in Thermodynamic equilibrium. Classical neither a pressure (state variable) nor a temperature (state variable) can be defined for this system. This is because pressure and temperature are intensive properties. If you think this can be done please indicate how since the values must include those of chamber B. Yes a volume (state variable) can be defined since it is an extensive property. Scenario (2) The system comprises the contents of chamber A alone. Chamber B forms part of the surroundings of the system. Now both temperature and pressure variables may be defined and the system is in Thermodynamic equilibrium, since its contribution to extensive properties need not be considered. Your internal partition wall is not now part of the system but part of the system boundary. When it is removed, the system looses its isolated status and mass is exchanged with the surroundings in the form of chaamber B. Note this is no different from considering a chemical reaction in which gas is evolved and collected in rubber (ie flexible) balloon. In this case the evolved gas 'pushes back' some of the atmosphere and some volume is incorporated in the system. Work is done and needs to be considered in the energy balance of the system. Another way to put this is to say that the system has (been) changed. As far as I can see scenario (2) concurs with what Timo said, but scenario (1) does not. What exactly is your understanding of the relationship between work and entropy change? -

Variations and consequences of the Laws of Thermodynamics
studiot replied to studiot's topic in Engineering
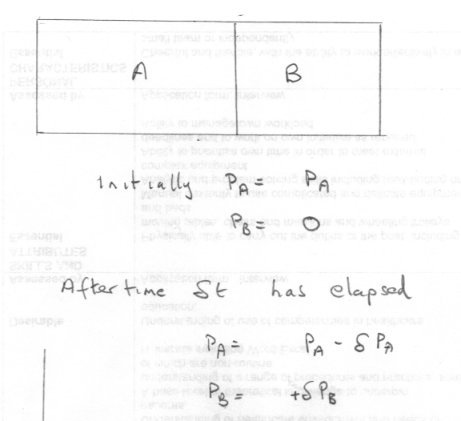
What about the work done in the expansion? "No work has been done" Well look at this Call the chamber with the gas A and the empty chamber B so the pressures are Initially PA and PB=0. Now however quickly some gas transfers itself, it is not possible for the final pressure to be achieved instantaneously. It takes time for the pressure to equalise throughout the combined chamber. So the pressure in A is constantly declining and the pressure in B is continually rising, both towards the equilibrium condition. It is true that as gas expands into B initially it faces zero pressure But As soon as a small amout of gas has transferred, at time δt, the pressure in B is no longer zero but some small positive value. Now any further expansion will be against this (albeit small) positive pressure so work must be done. Of course PA is now diminished and continues to do so. As this process continues ie in the next δt, this work will increase as the pressure in B rises. -
Well I thought for a moment that the OP was going to explain his space moves thing around as his version of what happens as a result of the expansion of the Universe, which might even be supportable But then he introduced Harry Potters magic nanobots and I realised that what he really meant by A small problem with the whole of physics was that physics should have been spelt with a capitol P. Oh and +1 to Zap
-

Variations and consequences of the Laws of Thermodynamics
studiot replied to studiot's topic in Engineering
All states are "equilibrium states". A condition of non equilibrium is not defined/definable. This arises because the full description of a state comprises a list of the values of all state variables, each of which which must by definition represent the entire system. -
Isn't this a contradiction in terms? What do you actually mean?
-

Variations and consequences of the Laws of Thermodynamics
studiot replied to studiot's topic in Engineering
Thank you for your contribution Timo, you poneyed up with many of many underlying thoughts, in particular that Thermo can get quite tricky when you start to think deeply about it. What you have said deserves some serious thought and consideration and may well lead to a better version of what I am saying. -
That is quite a lot of data to be processing. What subject and level is this an assignment in? You say you are using polynomial of 6 for graphing. Why does this mean and why are you using it? You have 12 estimates of a time series analysis showing both continued upward growth in cases and a peak and decline of cases in the middle years. The first thing I would do is to try to plot these as 13 ordinary number v time graphs. This would give you an envelope within which you can create your model. There is also the variation across the months to consider which can be done as a separate exercise. +1 to prometheus who posted whilst I was thing on this and clearly beat me to much the same ideas.
-
Because of all the fuss I looked through your thread and I must say I found it boring as hell, with nothing to reply to at all - so I didn't. This often happens. Uninteresting threads wither away through lack of support. Others thrive for opposite reasons. On the other hand, the fact that so many members have been able to put their views here in this thread shows what a fair minded forum this is. I don't know of another (scientific) forum that would have allowed such a lengthy free debate as this. So consider yourself lucky you are a member and follow the rules here and the guidance from those who have been here (much) longer than yourself.
-

Variations and consequences of the Laws of Thermodynamics
studiot replied to studiot's topic in Engineering
Yes really. Agreed, but you are focusing on the wrong part of that sentence. Again agreed, but a state is not defined by one variable alone but by the inclusion of every state variable. Other state variables are also different between the new and old systems. I meant (and said) that the irreversible process has changed the system. So the state of the new system (being a list of values of all state variables) differs from that of the original system. Consider two conditions on the original system. In one case the original sytem is truly isolated. In the second case the original system is not isolated an so can interact with its surroundings. In the second case and following the removal of the internal wall it is possible to restore the resulting system to the same conditions (list of state variables) as pertained in the original system, by outside intervention or if you prefer interaction with the surroundings. So it can truly be said that a system in state A can be taken to state B and returned to state A as the same system, but with changes in the surroundings. It is the emboldened phrase that is the usual way of putting this, but that is all too often ignored. However with the isolated system interaction is barred so the return is not possible, and would still not be possible regardless of the entropy issue. Entropy is a measure of an observational truth, not the other way round. -
Fragmented curve we are geeting further and further away from your original intention. My apologies, but that is the price you pay for posting in a fundamnetal science part of the forum rather than applied (engineering). Perhaps I should have offered a more down to earth response. Yes we can cool the air, and since in removing some heat, that heat has to go somewhere some of it could be converted to electricity. But whether your idea is viable is an engineering question, not a fundamental science question. Fundamental science in the guise of thermodynamics tell us that we need to spend energy to remove some heat from the air. I have a heat pump which does just this, but it is run by electricity. At this time of year the heat I get out is equivalent to a little over 3 times the electrical energy I use in the machinery. But the heat I get out comes out in the form of hot water not electricity. It is theoretically to use this hot water to drive an electrical generator, but I would get significantly less electrical energy that the heat energy I use. And I want the hot water. There is no such thing as a free lunch in this universe. If you wish to continue the discussion in these less esoteric terms please come back to us.
-

Variations and consequences of the Laws of Thermodynamics
studiot replied to studiot's topic in Engineering
It's more important and more subtle than that. Your proposed experiment uses an irreversible process. This means that since the original system is isolated and remains so it cannot interact with its surroundings. But the only way to restore the system to its original state is by way of an external intervention or interaction. In truth the original state is not accessible to the new system after the irreversible process. This is because there are really two systems the original system and the new or transformed system. The state of either of these systems is inaccessible to the other. -
My apologies I have spotted the glaring error. The transitive sentence should read "1) Then if Z1is in equilibrium with Z2 and Z3 is also in equilibrium with Z2 then Z1 is in equilibrium with Z3 ( This is the transitive property). " But please note I didn't originally think it useful to put it in this manner. The other simpler properties are actually deductions from it or consequences of it. Have you heard of the equipartition theorem? http://vallance.chem.ox.ac.uk/pdfs/Equipartition.pdf
-

The Classical (relativity)/Quantum Divide has been solved? Q ≤ 2D
studiot replied to hipster doofus's topic in Speculations
Yes but there are two states available. The particle is then merely the vehicle for the superposition (of states). The particle itself is not in superposition with anything. Superposition is not possible for a single entity.- it is not a reflexive property. Superposition of 'particles' occurs for instance in the spatial superposition of electron orbitals (for electrons) I even gave a formula for it (which Doofus is steadfastly ignoring) it is called the linear combination of atomic orbitals or LCAO method. Well I have used the superposition method of calculation many times in structural engineering. It is also widely used in electronic circuit theory and other branches of engineering. So widely in fact that it appears as a standard method in any standard textbook. -

The Classical (relativity)/Quantum Divide has been solved? Q ≤ 2D
studiot replied to hipster doofus's topic in Speculations
In superposition with what exactly ? You are simply confirming my earlier suspicion that you have no idea what the term means, either in QM or elsewhere, and just using it because it sounds good. -

The Future of the Scholarly Peer Review – A Road to Mediocrity?
studiot replied to Will9135's topic in General Philosophy
Statistical analysis was only one example. In my view the review panel should have as wide experience as possible, Perhaps that way a proper trial of Thalidomide might have been made and all those tragedies avoided.