-
Posts
18270 -
Joined
-
Last visited
-
Days Won
104
Content Type
Profiles
Forums
Events
Everything posted by studiot
-
I asked you to explain your opening post because, as I siad, I was not sure what you were after. Reading your replies to myself and others I am still not sure, although I now have a list of what you are not seeking. So please explain your opening post. You never know, you may be pleasantly suprised.
-
I am not asking "how to put numbers to the time axis." Are you serious? Read and comprehend the first sentence in the OP, then reconsider, please. My question requires one to know the nature of 'time'. I didn't comprehend the first sentence, and I said so. So please don't be insulting, just answer my question.
-
I'd be content just knowing how to figure it for one abstract molecule without the complications of the entire atmosphere. Reinventing wheels teaches me how to make wheels That's a helpful link and I will have to study that more. The last comment on the page tipped me off to Stefan-Boltzmann which allows me to describe power in terms of area of a blackbody, which is what co2 is at a certain frequency. At least I can say "this many watts goes to space and that many heads to earth", but first I have to decide how much area a co2 molecule has and what T will be. The Beer's law requires an optical depth, so I'm not sure that applies here. I'm still struggling to learn the nomenclature: Roughly, the temperature of a body at rest is a measure of the mean of the energy of the translational, vibrational and rotational motions of matter's particle constituents, such as molecules, atoms, and subatomic particles. The full variety of these kinetic motions, along with potential energies of particles, and also occasionally certain other types of particle energy in equilibrium with these, make up the total internal energy of a substance. Internal energy is loosely called the heat energy or thermal energy in conditions when no work is done upon the substance by its surroundings, or by the substance upon the surroundings. https://en.wikipedia.org/wiki/Thermodynamic_temperature That looseness is what makes this difficult and the last thing I want to start doing is associating heat with stored energy. Maybe the best way to attack this is to make a spreadsheet of every term (heat, internal energy, thermal energy, radiance, spectral radiance, radiant flux, temperature, thermodynamic temperature, yada yada) so I can have it all in front of me to make sense of. The math and the concepts are straightforward, so the language is the only barrier. Huh? Why do you want to confuse me like that? lol! How is light from a laser not heat? How is a fluorescent light doing work? I'm getting a variety of answers: 1) In thermodynamics, heat is often contrasted with work: heat applies to individual particles (such as atoms or molecules), work applies to objects (or a system as a whole). Heat involves stochastic (or random) motion equally distributed among all degrees of freedom, while work is directional, confined to one or more specific degrees of freedom. https://en.wikipedia.org/wiki/Heat 2) Work is any energy transfer that does not carry entropy. Heat, on the other hand, is any energy transfer that carries entropy. 3) Work is the mechanical transfer of energy to a system or from a system by an external force on it. Heat is the non-mechanical transfer of energy from the environment to the system or from the system to the environment because of a temperature difference between the two. The conversion of mechanical energy (work) to heat is very efficient, nearly 100%. But the conversion of thermal energy (heat) to work is not so because heat is a low grade energy. https://www.quora.com/In-thermodynamics-what-is-the-difference-between-work-and-heat-I-was-thinking-about-this-when-studying-Carnot-Cycles-and-adiabatic-processes-How-can-a-system-do-work-in-isolation-What-is-it-doing-work-on-Where-is-that-energy-going I like this one: 4) You are right. Microscopically, work and heat are just about the same. Both involve molecular collisions transfer energy from one object to the other. Work involves a kind of "coherent" transfer in a manner of speaking, in which the collisions are predominantly, and to an extreme degree, in one direction. Also, typically the force is applied to one location on the object. And importantly, the boundary of the system is displaced. (E.g. translation or deformation) On the other hand, transfer of energy by heat is "incoherent", many directions, and typically in all directions. And importantly, the boundary of the system is not displaced. Finally, everyday phenomena fall into one or the other category, and they differ in their macroscopic behavior. Loosely speaking, when heat is transfered the temperature rises. When work is done, the boundary of the system changes. Of course adding heat generally also results in the boundary moving [say, expanding], and work generally results in a temperature change [Joule's experiment]. I'm trying to motivate the macroscopic separation between work and heat without a lengthy discussion. To the engineers who first worked all of this out, the very existence of atoms was unknown. To them, the separation between heat and work was very clear. They had little reason to view them as manifestations of the same microscopic process. They thought that heat was a physical fluid. In any event, their remarkable achievements have stood the test of time. https://physics.stackexchange.com/questions/135539/why-work-w-and-heat-q-are-different-concepts What do you think? What's the best way to write distinct definitions for work and heat? Regardless how we excite the atom (photon or collision) it is still resonance that determines the energy level of the electron, right? So it's the mass and bond strengths that determine the energy levels. I don't understand why that would not be correct. Here is what I said before: It seems like it would be the resonance of the atom that determines the point where a photon would be absorbed and that would define the difference in energy levels. Not the other way around, which is the difference in energy levels determines if a photon is absorbed and that defines the resonance because it doesn't make sense that way. It's putting the cart before the horse. Right, as a function of mass and bond strengths which determine both resonance and energy levels. Well, I wasn't done replying to your post as I wasn't done studying it in hopes of replying with something that would be to your satisfaction. Not at all and I have no idea how you came to that conclusion as I took special care to prevent anyone from coming to that conclusion. Anyway, I've discovered the limits to my patience for walking on eggshells only to still be incapable of making you happy, so whatever. I'll figure it out with or without your help and if having your help means the continual negotiation of derogatory implication then I'd prefer to go-it alone. Thanks for trying. I really don't know why I am bothering but I will spell it out in simple sentences. You have asked a Chemistry question in the Chemistry section of this forum. You insist in the above quoted definition of Resonance. I have not told you this is incorrect. I have told you that your definition is appropriate to the Physics of Mechanical vibrating systems. I have also told you that there is a separate, very different definition in Chemistry. I have also told you that this usage has nothing to do with vibrating systems. In fact it applies to the carbon dioxide molecule whether it is vibrating or not. You have steadfastly refused to discuss this. In these circustances I can only suggest adding Chemistry to John Cuthber's list for you to study. I have also told you that the vibrations in question are not due to the promotion of electrons to higher energy levels. Again in your responses you steadfastly continue to promote this fiction, as with the quoted response to swansont.
-
You seem to put great store by your personal definition of classical and appeared to me to want to discuss this only in classical terms. But like most boundaries I think this one is blurred as well, where do you place retarded potentials etc? That aside I agree with your comment in general unless you are thinking about Force. I say this because you go on to say Which is not the situation I mentioned. (electric) forces are not exerted on points. The concept is meaningless. (electric) forces are only exerted on other charges, and I specified "there is only one charge". Please explain the connection. The use of the word particle implies zero dimensions. In those circumstances the mass particle does not attract itself. In the case of a distributed mass, we use the position and motion of the centre of gravity to determine mechanical output such as work, kinetic energy etc. However this is missing my point as this was just a comparative example. I also said that my analysis required an additional agent holding one charge in place, instead of relying on an overwhelmingly large mass difference as in the mechanical case. It should be noted that if this agent does not exist then Earnshaw's theorem requires both charges to be in eternal motion. Anyway I don't want to just quibble I would like to find out the point of your thread.
-
Yes I noticed so hopefully you understand the significance of the separation of charge But sadly I can only conclude the following from this address as the only response to an explanation, already twice given:- I take this last bit to mean "My mind is made up so don't confuse me with the facts"
-
Classically it doesn't work like that. The underlying classical equations for this are Coulomb's law which whould be compared with Newton's law of gravity. Both of these rely on at least two agents to generate an output. If there is only one mass or charge, then although there is a notional field, the force at all points is zero. So no work is done if we move that mass or charge about. The force and work only arise when we introduce a second charge or mass. In the case of gravity we normally consider the Earth so massive by comparison with the test mass that all the force and work is considered to be applied to the test mass alone, with the Earth considered immovable. But really we should be considering the centre of mass of the system to get the full picture. Because we disparity between the agents for charge is so much less, when we consider Coulombs law we (have to) introduce an external agent to hold one charge still. Then we can attribute all the effects to the other charge. Equally we could calculate the resultant field and the centre of charge and work that way. No charge will 'see' a different field, they all 'see' the same one. There is an equivalent magnetic theory which has stalled in the absence of magnetic monopoles.
-
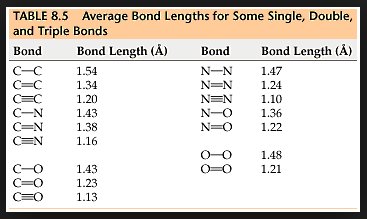
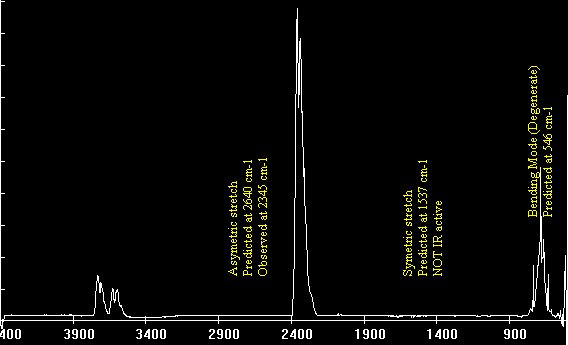
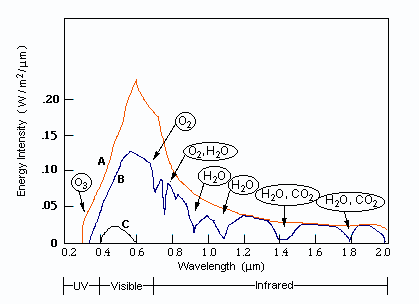
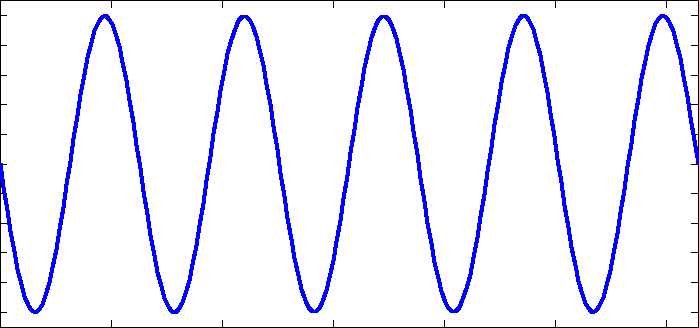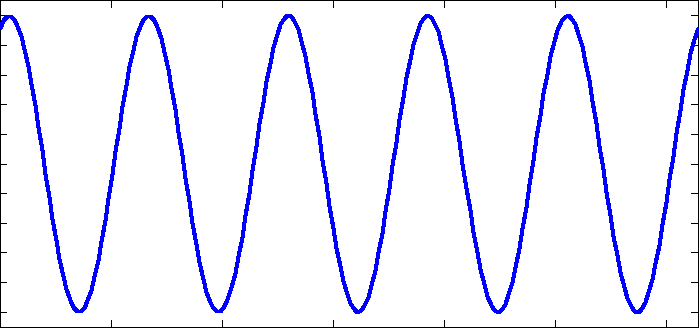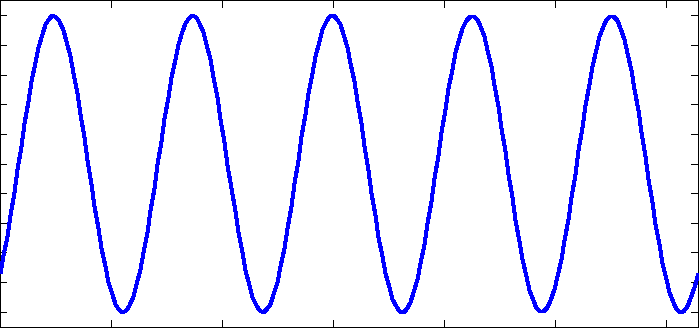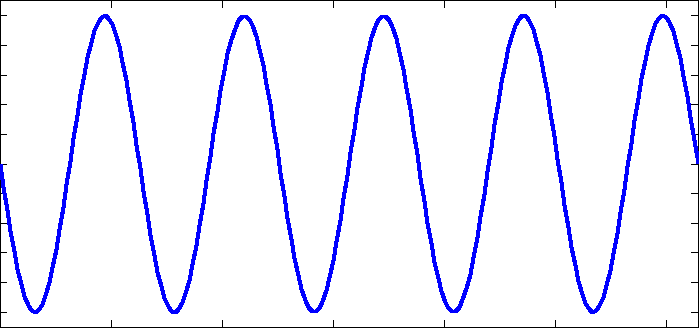
I am going to be away for a few days so before I go I will respond to some of your comments as promised. I really suggest you take the time to read them more fully as they contain information you are clearly missing. I don't understand "phase charge separation". That would be because you misread my text. I didn't say phase charge separation, I said the phrase charge separation. I can only assume you understand the meaning of charge separation. Not just bond length and strength, but mass as well. Right? What I failed to see before was that EMWs only interact with charges, not matter itself. So the charges will be the "handles" that the EMW "grabs" to then shake the molecule and how the molecule behaves is determined by the bonds and the mass of the atoms. Yes mass also plays its part, I was going to go on to present the formula that results for the carbon dioxide molecule along with the expected bond lengths and frequencies, but you 'jumped the gun'. Note that each my explanatory notes is there for a reason so when I recommend forgetting resonance I also offered a reason so I was suprised you did not ask for this rather than your actual response. I can't forget resonances. For instance when an EMW causes co2 to resonate, it absorbs energy which is expressed by the exaggerated movement and an electron being kicked up to the next energy level. Resonance makes too much sense to me. Here is a list of 'normal' bond lengths. Note that the actual measured length in carbon dioxide is 1.16 Angtrom, a significant shortening on the 1.23 shown above. Chemists attribute this to the three resonance structures of carbon dioxide and the resulting so called resonance energy. Again I was going to discuss this in more detail until you rejected it. I'm a little confused how the co2 molecule would interact with the microwave band because: Often molecules contain dipolar groups, but have no overall dipole moment. This occurs if there is symmetry within the molecule that causes the dipoles to cancel each other out. This occurs in molecules such as tetrachloromethane and carbon dioxide. The dipole-dipole interaction between two individual atoms is usually zero, since atoms rarely carry a permanent dipole. https://en.wikipedia.org/wiki/Intermolecular_force The way I understand microwave heating with water is that the MW rotates the water molecule which then interacts kinetically with other molecules that cause resonances in the water molecule in the IR band. Water is a dipole, but co2 is shaped more like - + - so I'm curious if MW could rotate the molecule in the same way. Right so the plan was to show the link between the charge separation that occurs in a radio aerial and the charge separation in a carbon dioxide molecule and how they are similar. I have already told you that the vibrations concerned are stretching and waggling modes. You are correct that carbon dioxide has no dipole moment on symmetric stretching (but does have a qudrupole moment) so no absorbtion band there, but it does have an asymmetric stretching mode, associated with the resonance structures. It also has two waggling modes that are degenerate. Degenerate means they have the same energies because they are simply the same waggling motion in two planes at right angles. Carbon dioxide is a linear molecule, unlike water. So all the molecular rotational modes appear as fine spectroscopic structure on the bond distortion modes. None of the electron promotion energies correspond to IR or microwave regions. If we continue with the plan we can explore pictures/animations of this next time. Meanwhile I will leave you with an picture of an actual carbon dioxide spectrum readout showing the three main modes and the 'missing' symmetric mode. And also some spectra from various molecules in the atmosphere, that may prove useful later.
-

Does the sun release stored energy?
studiot replied to MarkE's topic in Modern and Theoretical Physics
I think we need to look more closely at some of the words you are throwing about. This is because although these words are derived from English they have particularly tightly defined meanings in Science which are quite restricted compared to their general English usage. So to start with a region of space. This is pretty much the same as its English counterpart and might be the inside of an eggshell or a smartie box. More usefully in Science it might be the bore of a pipe or the interior of a chemical reaction flask. Or of course it could be the whole of space itself or just the space around planet where the panetary gravity is not negligable. Whatever region we take it, we need a boundary to describe what lies inside the region and what lies outside. Proper specification of that boundary is of vital importance in Science, particularly Thermodynamics - the Science of Heat. We also identify every individual location or point within the region. Some of these have special names such as the centre of gravity. Another English word with a special meaning in Science is the word Field. In English it often means 'the stage in which the activity takes place' eg the 'field of operations', 'he was an expert in his field' and so on. This meaning is not taken on by Science, so do not use it in a scientific manner. In Science a Field is a region of space where every point of that region has a specific value of some property of interest assigned to it. Every point might have the same value eg the density in a homogeneous substance, or the pressure inside a small balloon. So we can talk of a density field or a pressure field Or the value may be different at every point for example the temperature field within a large copper bar, heated at one end with a blowtorch. The properties of that Field as a whole will depend upon the particular property we are considering. Some of these properties as simple numbers like in my examples, some are much more complicated mathematical expressions, such as the electric field vector in an electric field or the magnetic field vector in a magnetic field. But there may be many properties and other things (like machinery or electric fires) inside a region. In themodynamics we call such a region a 'system' and we include, but separately identify, the boundary. These concepts allow us to develop conservation laws. Conservation of mass, conservation of momentum, conservation of energy. OK, so I used the E word - Energy. For the moment, I will simply observe that there is only one property called 'energy' that has many forms that are all equivalent and stop there to allow you to digest this lot and ask any questions. -

Wave particle Duality inspired by a thread in Chemistry
studiot replied to studiot's topic in Quantum Theory
-
My apologies too. I have corrected the units oversight on my last post. milliMole -seconds should have been milliMole -minutes.
-
Yes it is an old post and you should have started your own thread for an unrelated question. So don't be suprised if a Moderator splits this off. Since you are studying a biological science subject you should have posted there for a biologist to reply. I think you are making too much of the whole issue and confusing yourself. A hundred years ago or so scientists drinking brandy in their fashionable clubs realised that most, if not all, the quantities used in science could be built up from a few basic ones. There are many possible selections of basic quantities to choose from so they had more brandy. After a few more brandies they established the beginnings of the current system as the most convenient. This is the one you are using today. Those combinations of special importance or interest have received special names in their own right, for example energy. Some combinations are, to put it frankly, not important enough to warrant their own name In that system the metre is represented by a small m. A large M is used for Moles - a chemistry term. Unfortunately there are more quantities than letters, even with the greek and other alphabets included. So m also represents the prefix 'milli'. So I would guess the units of glycemic response to be milliMole -seconds edit milliMole -minutes
-
I am sorry I rather scrambled my last post so you missed the main point which was that you don't only have to consider the lance length you must also consider the shrinking empty space between the jousters.
-
You are correct the answer to the OP probably has to do with the temperature of the water. Asthfx perhaps you live in a part of spain where they don't have ice? If the same mass is squashed into a smaller volume will the density go up or down? I have underlined you proposed answer. Chapin if you define % change you will see that a positive tells you the density has increased, but a negative change tells you that it has .................? Do you know that water is an anomalous substance? For most substances density increases as temperature falls and this is true of water between 100oC and 4oC but water has its maximum density at 4oC and the density then decreases between 4oC and0oC. When water freezes it expands so ice floats on water, unlike most solids on their respective liquids.
-
I have started a new discussion about wave/ particle duality in the quantum theory section http://www.scienceforums.net/topic/110124-wave-particle-duality-inspired-by-a-thread-in-chemistry/ I will address the (useful) remarks by BanterinBoson in a second reply.
-
Discussion of quantum theory is getting in the way of discussion about chemical spectroscopy in this thread and we all agreed that it would be better conducted in a separate place. As this forum has an allocated palce for quantum theory I am starting this thread to promote that discussion. http://www.scienceforums.net/topic/109814-vibrational-frequency-co2-global-warming/?page=2&tab=comments#comment-1014291 Here is a kick off post by BBoson by way of explanation. Let's save that for other discussions I agree that quantum mechanics is quite different from classical mechanics. Like classical mechanics, QM can be approached at different levels from different viewpoints. One such is using 'wave packets' to describe duality A classical wave extends to infinity in both directions. Mathematicall the classical wave equation has no beginning or end. We simply ignore that part of the mathematical equation outside our region of interest. A wave packet has a beginning and an end and can be used as a model as to how you can have wave/particle duality.
-
I think the key to this one is to realise that not only does the each jouster see the approaching lance as shorter than his own but he also sees the distance between himself and the tip of the moving lance to be shorter. That is he sees all distances in the moving frame (considering himself static) shorter along the inter-line.
-

Does the sun release stored energy?
studiot replied to MarkE's topic in Modern and Theoretical Physics
The answer should be that I have given you some new terms for you to look into. There is a more modern explanation in particle physics, without forces at all, that Swansont and Sensei are offering, but this is a good route there, following the historical development of the subject. -

Help needed arguing with a creationist
studiot replied to DrKrettin's topic in Evolution, Morphology and Exobiology
There is more to it that that. Is an aerobic microorganism more complex than an anaerobic one, or just different? The original lifeforms were all anaerobic since there was no oxygen to start with. In fact oxygen was a poison to them, to be excreted by them. -

Does the sun release stored energy?
studiot replied to MarkE's topic in Modern and Theoretical Physics
Here is a simpler summary of one of our models that best moves on from what you already seem to know. Yes potential energy is stored in the arrangement of atoms within a molecule. This energy is electrostatic in nature as is the force that generates it. You are right in observing that this force opposes the cramming together of like charges as we find in the nucleus. So there must be other forces involved and your question seems to be about what these are. Furthermore these forces must be stronger than the electrostatic ones. We identify four what are called fundamental forces. 1)Gravity 2)The electromagentic force 3)The strong nuclear force 4) The weak nuclear force All four lead to a potential which can be regarded as an energy store. Early physicists discovered that the strengths of forces (1) and (2) vary with distance, the stength falling away as distance increases. They also found that the electromagnetic force is many orders of magnitude greater than the gravitational one. When physicists asked the very questions you are now asking they realised that the forces holding the nucleus together must be very short range compared to the first two. So these forces really only act within the atom. So just like the arrangement of atoms in a molecule, the arrangement of sub atomic particles in an atom can be regarded as storing energy in the potential fields of (3) and (4). As already noted the internal electrostatic field within the atomis small by comaprison . This energy is known as binding energy. Now observation has shown that the binding energy per nucleon varies with each atom. This is shown by what is known as the packing fraction curve. And this is interesting because this curve has a minimum at atomic number 56 (iron). This means that if nuclei smaller than iron are combined energy could be released until the resulting combined nucleus is that of iron. We call this fusion. Equally nuclei larger than iron can release energy by breaking apart to move towards nuclei of the size of iron. We call this fission. As you say there is a thermodynamic imperative for this, but it is due to energy not entropy. As with all thermodynamic calculations we work this out by adding up the sum of all the potentials of all the species before the process and comparing that with a similar sum for the species after. This is how fission can release much smaller particles than iron as well. There is a net energy release. As a matter of interest, I think but I am not sure, that our Sun is not hot enough for even helium to fuse, that happens in a nova. And to get all the way from hydrogen to iron to need a supernova. -

What causes to 3rd law of motion in theory of Newton
studiot replied to spiderweb's topic in Classical Physics
If you want to become an expert on Newtons Laws I would recommend you follow the standard notation. Otherwise I) You will fail exams when they ask you to use N2 to prove something and you use the wrong law. 2) You will stumble over communications with others as you have just done with me. 3) You will come across more difficulties when you start to discuss the application to circular motion. Now would you like an answer to your question about Netwon's second Law, N2? -

What causes to 3rd law of motion in theory of Newton
studiot replied to spiderweb's topic in Classical Physics
Let us start with a correction because you have stated Newton's second law not his third. So did you mean really mean N2 not N3 as they are called for short?


.gif.8603d262ff8b16958236022c16e6aa3a.gif)