-
Posts
18258 -
Joined
-
Last visited
-
Days Won
104
Content Type
Profiles
Forums
Events
Everything posted by studiot
-
This curve has some remarkable geometric properties as well as calculus ones. Calculus had not been invented when Descartes discovered it in 1638. Jacob Bernoulli had one engraved on his tomb along with the words "Eadem metata resurgo" Because of the constant angle property, if you roll the curve along a straight line, its centre or pole describes another straight line at an angle to the first. Erect a perpendicular from the pole to the first straight line and the length from where it cuts, back along back the first straight line to the starting point is equal to the length of the spiral.
-
It would be more fun to solve the way Descartes originally did.
-
Why does your diagram show an r but your wording a d? Is this intentional misdirection?
-
Good point +1
-
That is a really good and important question for the 21st century that no one knows the answer to. I assume you mean continuous in the mathematical sense. This question goes to the heart of the difference between quantum theory and relativity/classical physics. A pity you added this about fudges, which is all such speculation amounts to. We do not have enough information to answer this.
-

[Thermodynamics] Macrostates - Volume, Moles, Pressure, Temperature
studiot replied to imdow123's topic in Classical Physics
You have posed a very deep question, but your attempt at answer is insufficient. Thermodynamics is much more complicated than that, and I thought you wanted to go on to deeper things. That is why I have been trying to find the level to pitch a suitable answer. For an single phase as a single component ideal gas in equilibrium only Temperature can be derived from a PV diagram. That is because for this system every point on the diagram corresponds to a unique state and thus a unique calculation according to the ideal gas law can be made. Now if the system is not in equilibrium it cannot be plotted on a PV diagram. Further if you have a PV diagram for water there are points on the diagram that will not return a unique temperature, even when the water is in an equilibrium state. -

Course preparation for Computational chemistry
studiot replied to just_starting_out's topic in Applied Chemistry
Statistics and probability is the main area that stands out as missing from your list. Intuitive Biostatistics Harvey Motulsky Oxford University Press Provides a first class base to build on in this area. Take a look at The Chemistry Maths Book Erich Steiner Also Oxford University Press This covers all the areas needed in undergraduate chemistry in a highly accessible format. You want to extend beyond undergraduate level. Chemistry these days is beginning to take advantage of group and symmetry theory and the more abstract aspects of university level alegebra. -
Not necessarily, but your formulae or mine will always work. eLg presented a problem in the (mathematical) 4th quadrant. Don't forget the sign rules for different quadrants. Your formula is quite correct, but the sin and cos are reversed for normal surveying or navigation calculations, since they are measured from the North line, not the east line. The correct terms are northing (y) and easting (x) or latidudes (y) and departures (x) http://www.cfr.washington.edu/classes.esrm.304/Spring2011/Documents/Hurvitz_Schiess/procedures/latitudes_and_departures.html
-
I think this should be angle = - (theta - pi/2) Otherwise you will get the wrong sign (and answer). You can also use angle = (450 - theta) if you do not wish to use negative angles.
-
Do you not think this classical? It was first proposed by Democritus a few hundred years BC and revived by Dalton around 1800 AD. Various other 19th cent scientists confirmed the discrete nature of charge as well as matter, during the course of that century.
-
But the work is not negative. This is just about sign conventions. The expression of the First Law given is dU = Q-W so corrresponds to my first sign convention in your previous thread. Work done by the system (aluminium block) is positive. The quantity in brackets in the example is clearly positive and subtracted from the incoming heat, in acordance with this First Law convention. Think of internal energy as like your bank account with receipts as the heat and payments as the work done. Both are positive. The First Law of Banking says Closing Balance = Opening Balance + receipts - payments. Does this help?
-

[Thermodynamics] Macrostates - Volume, Moles, Pressure, Temperature
studiot replied to imdow123's topic in Classical Physics
I'm sorry, how was this a response to my post#11? I have stated specifically that what you ask is not possible, so why repeat? I need to know whether you have heard of the Zeroth Law or Gibbs Formulation or generalised coordinates to discuss this. -
What exactly did you not undestand about this?
-
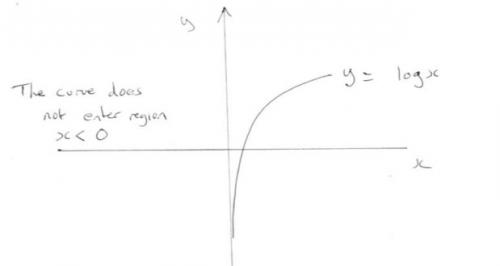
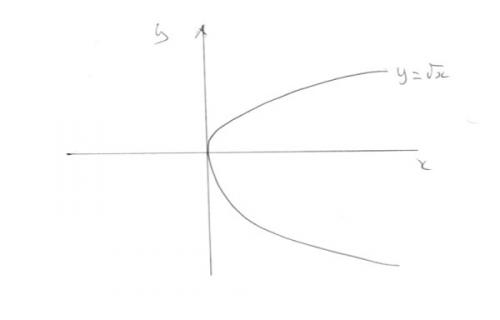
Draw a graph of y = log x (any base) Note that the graph does not cross the y axis or it does not enter the region where x<0. All values of the log graph appear on this line and nowhere else on the graph. This is the same as for a square root. Are you suprised there are not square roots of negative numbers? This is true of all graphs. They show the only values of the expression.
-

[Thermodynamics] Macrostates - Volume, Moles, Pressure, Temperature
studiot replied to imdow123's topic in Classical Physics
Without the temperature variable, how would you solve the following problem with all the information given, except the temperature rise? That is you are given the number of moles, the pressures and volumes exactly as in the problem. http://www.scienceforums.net/topic/79099-thermodynamics-example-with-answer/ This is the simple practical answer. We can discuss a more esoteric one if you like. The Zeroth Law of Thermodynamics requires the existence of a temperature state function. This can be proved using the Gibb's Formulation of thermodynamcis in terms of generalised co-ordinates. -
What methods, other than factorisation do you know for solving quadratic equations?
-
OK that's a worry that trips many people up when they start thermo. The First Law is basically the Law of Conservation of Energy flows across the system boundary. Energy in adds to the internal energy. Energy out decreases it. Since energy can flow in either direction we need a sign convention. Unfortunately there are two sign conventions. The original convention, used mainy by engineers and physicists is Work done by the system is positive (= work out = energy out) Heat added to the ssytem is positive (= heat in = energy in) This leads to the First Law in the form dU = q - w Which is the form at the bottom of your example. The second convention, used by chemists and many scientists counts all types of energy in as positive and all types of energy out as negative. Heat added to the system is still positive but Work done by the system is now negative. This leads to the First Law in the form dU = q + w You should always check the sign convention in use. Note this advice applies in other areas of physics that also have different possible sign conventions. Does this help?
-

[Thermodynamics] Macrostates - Volume, Moles, Pressure, Temperature
studiot replied to imdow123's topic in Classical Physics
I have extended the discussion in post#7 Please ask questions, I'm sure there will be some. -

[Thermodynamics] Macrostates - Volume, Moles, Pressure, Temperature
studiot replied to imdow123's topic in Classical Physics
Since you are online at the moment, I started an overview, but have not finished it yet. Here is the beginning, let me know if you would like me to carry on. Here is an overview of thermodynamics and the relationship between statistical and classical thermo. Thermodynamics divides the universe into two parts by a specified boundary. The part of interest is called the system and the rest of the universe is called the surroundings. Any particular thermo problem can be made easier or more difficult or even impossible by appropriate choice of boundary i.e. what we choose to include or exclude in our system. Properties of interest are defined by variables. Variables are called extensive if they are proportional to the amount (mass) of matter present and intensive if they are independent of mass. Some variables are neither extensive nor intensive. Some variables are directly observable (measurable), some have to be deduced from various available relationships that exist between variables. Conditions within a system may be static or most often they are changing. This presents two immediate difficulties. Firstly for intensive variables we have one value to represent the whole system, however unless the system is homogenous and isotropic the value varies from point to point within the system especially as changes occur. Examples are temperature and pressure, two important directly observable properties. Note with extensive properties no such difficulty exists since we can take the value over an (infinitesimally) small region and sum to obtain the value for the whole system. It does not matter whether there is local variation from point to point the answer is still the same. So we can add up all the mass points or heat capacities of a system to find the overall value. I will return to the second difficulty after discussion of the boundary. Whilst classical thermo, in conjunction with kinetic theory, offers some relationships between some of the variables within a system, for example the gas laws, it mostly concentrates on exchanges across the boundary. So the First Law is concerned with energy that crosses the boundary in the form of work, heat, change of phase etc. Statistical thermo, on the other hand, is not so much concerned with the source or flows of this energy, but with its distribution and redistribution within a system. Boltzman’s Law being its crowning glory. Energy flow across the boundary is another example of directly measurable quantities. In order to know about the system we define a condition known as a ‘state’. We can do this if we can allocate values to ‘variables of state’. These include Pressure, Volume, Temperature, and a number of derived (calculated ) variables such as internal energy, entropy and so on. Variables of state are such that the change in the value of a variable of state from one state to another equals the difference in its value in each state. Work and heat flow (cross boundary variables) are not variables of state. You can immediately see that this leads to the second difficulty. If for instance the pressure in a gas is not homogenous you do not have a single value to insert for pressure in relations relating pressure to another quantity. The same goes for temperature. The non linear nature of most of the formulae mean that averages are unsuitable substitutes. So classical thermo has devised several prongs of attack on this difficulty. Firstly for an infinitesimal change we can regard all properties as unchanged. So if we can find and integral formula and integrate it we can still calculate. Secondly the idea of the cyclic process. If we return the system to its starting point we can find formulae for the net effects in terms of the cycle. Much classical theory takes place in terms of cyclic processes. These are unnecessary in statistical thermo. Thirdly we can sometimes find alternative expressions for a quantity. For instance the work done across the system boundary. It is obvious that the work done by the system must equal the work received by the surroundings. So although we cannot directly calculate the work done expanding most gaseous systems, because we do not know the pressure at all times at all points, if the expansion is against say the atmosphere we can say this is sensibly constant and calculate the work this way. In order to properly specify and analyse a thermodynamic system we must specify The system components The system boundary The system process . -
Surely this question has been answered a dozen times now? Any primary school child should be able to answer this. Just write down the first number in decimal form, including the decimal point. Write down the secon number underneath, also in decimal form, aligning the respective decimal points. Starting from the right add digits in each column, carrying over to the left. The result will be a (perhaps very long) number in decimal form. You can, if you like, follow the simple rules to convert it back to exponent form. All other considerations are a distraction from the original question.
- 19 replies
-
-1
-
How is this dividing by x-3? What exactly do you mean by the left hand side of the original equation? What happened in your other thread this morning?
-

Prove convergence of a series of real data
studiot replied to AlexSaly's topic in Applied Mathematics
If in doubt - ask. -
Can you state the question in words? We can put it into maths symbols later. Its a requirement for everyone to take a calculus class for all subjects, not quite sure why. Many areas of university Chemistry require the use of calculus. As to why you are being asked this particular question, if the limit of a function, say f(x), as the function approaches some point say x=c equals the value of the function when x=c is substituted into the formula, the function is continuous at c. This is the condition for the function to be differentiable at c. Continuous functions and their differentiability are very important in science generally. A simple way to approach limits is to add a small quantity (h) to x and study what happens as h becomes smaller and smaller, allowing that 1/0 "tends to zero" as this happens. In this case you need to make a substitution first, so let [math]{r^2} = x[/math] and [math]{h^2} = c[/math] Now substituting for x and c in this limit [math]\mathop {\lim }\limits_{x \to c} \left( {x - c} \right) = \mathop {\lim }\limits_{h \to 0} \left( ? \right)[/math] can you see this is equal to [math]\mathop {\lim }\limits_{h \to 0} \left( {{r^2} - {h^2}} \right) = \left( {{r^2} - 0} \right) = \sqrt x [/math] I hope this was for basic understanding, not homework. Homework should be posted in the homework section where we get you to do the work.
-
You missed something out of the limit. I presume you mean [math]\mathop {\lim }\limits_{x \to \infty } \frac{{x - c}}{{\sqrt x }}[/math] [math] = \mathop {\lim }\limits_{x \to \infty } [/math][math]\left( {\left( {\sqrt x } \right) - \frac{c}{{\sqrt x }}} \right)[/math] I can't see you would need an epsilon-delta proof for Chemistry so what methods do you know for taking limits?
-
I would guess this is the culmination of an algebra course as it combines several facts about equations and their solution? How would you normally solve an equation with x in the power? There are some other steps to take to put this equation into a suitable form before you can take this action, you will need to change this equation into a quadratic to solve it,