Lazarus
Senior Members-
Posts
366 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by Lazarus
-

Do electrons radiate from electostatic acceleration?
Lazarus replied to Lazarus's topic in Classical Physics
Please point me to an experiment or apparatus that demonstrates the radiation. -

Do electrons radiate from electostatic acceleration?
Lazarus replied to Lazarus's topic in Classical Physics
Swansont said: Yes. Why wouldn't a single particle radiate? Reply: The electron in a synchrotron is accelerated by magnetic forces and it radiates. That doesn't necessarily mean that electrostatic acceleration should radiate. Do the electrons traveling towards the screen in a TV radiate on the way? -
Does an electron radiate while being accelerated by an electrostatic force or gravitational force? The posts here say it always radiates when accelerated. It has been demonstrated that electromagnetic force causes the electron to radiate. Is there an experiment that determines the result of electrostatic force? This item on the internet seems to contend that acceleration doesn’t always cause the electron to accelerate. Will the real answer please stand up? (You aren’t old enough to remember that show.) The physics Hypertxtbook 1998-2013 Glenn Elert X-rays are produced whenever fast moving electrons are decelerated, not just in x-ray tubes. Nearly all the naturally occurring x-ray sources are extraterrestrial. (No, that doesn't mean produced by alien creatures from outer space. It just means ''beyond the earth”). X-rays are produced when the solar wind is tapped by the earth's magnetic field in the Van Allen Radiation Belts. Black holes are significant sources of x-rays in be universe. Matter falling into a black hole experiences extreme acceleration caused by the intense field of the black hole. ???????????????????????????????? A single isolated particle would fall in without releasing any radiation, but a stream of particles would, as the particles would wind up crashing into each other on their way down the hole. ???????????????????????????????? Each inelastic collision experienced by a charged particle would result in the emission of a photon. Since these collisions are taking place at great speeds. the energies of the emitted photons are on the order of those found in the x-ray region of me electromagnetic spectrum. Inelastic collisions at even higher energies (greater than a million electron volts) would generate gamma rays.
-
Swansont said: Any of them. Dipole and higher-order multipole moments are known and measured for nuclei. You have to show that your model matches them. Reply: Sorry for the long delay in replying. Sometimes life gets in the way of fun stuff like this. Your insistence on relating the nuclear moments to the model was very helpful. Having the protons side by side seems to work better in most cases. The moments of two protons with the north south poles in opposite directions cancels out the magnetic moment. The easy part is nuclei that have even Z and even A. Almost any symmetrical configuration would work. The way that a proton and a neutron can become a hydrogen 2 nucleus is described below and in the diagrams that are linked. Since the difference in mass of the proton and the neutron is divisible into both the difference can consist of one positron and 2 electrons. A neutron consists of 1201 electrons and 1201 positrons. A proton consists of 1199 electrons and 1200 positrons. A neutron plus a proton totals 2400 electrons and 2401 positrons. A deuteron has less mass than the sum of the proton plus the neutron. The average loss is 2.84 electron/positron pairs. The loss consists of either 1 pair or 3 pairs. The proton has 3 humps. When a proton and a neutron are forced together to form a deuteron some mass is lost and the center hump shares 3 electrons and 2 positrons with the neutron which now looks just like the proton. The end humps share either 2 electrons and 3 positrons or 1 electron and 2 positrons. The rest of the deuteron has either 2390 electron/positron pairs with a loss of 3 pairs or 2394 pairs with a loss of 1 pair. The proton and neutron are “side by side” with their north/south poles in opposite directions. That allows the unmatched positron to produce the nuclear moment. Because the shared electrons and positrons are between magnetic forces in opposite directions there will be some distortion of the moments. When the atomic number is odd all the matched pairs of protons cancel out except 1 or more of the protons which contribute to the nuclear moment. When the number of neutrons is odd (so electron are odd) 1 or more of the electrons contribute to the moment. Even numbers can have 2 or more contributing to the moment. The tables at the end of this post show mass loss and moments for some of the elements. The following links are to diagrams than I couldn't get to insert for some reason. http://www.flickr.com/photos/111049597@N08/11281648674/ http://www.flickr.com/photos/111049597@N08/11281594223/ The following table shows how the moments can be approximated by the number of protons and electrons that add or subtract moment. Some of the calculated values are fairly close and some are not. The difference in orientation of some of the electrons could be responsible for the disparity. The electrons in the shared portion can't align north/south with both of the protons beside them. Calculated vs Book Moments Name Z e A Book Calc A e H 1 0 1 2.792 2.799 1 0 H 1 1 2 0.860 -0.880 0 -1 He 2 1 3 -2.136 1.919 1 -1 Li 3 3 6 0.820 -0.880 0 -1 Li 3 4 7 3.260 3.678 1 0 Be 4 5 9 -1.180 1.919 1 -1 B 5 5 10 1.800 1.880 0 1 B 5 6 11 2.690 2.799 1 0 C 6 7 13 0.714 0.159 1 -3 N 7 7 14 0.400 -0.880 0 -1 N 7 8 15 -0.282 1.039 1 -2 O 8 9 17 -1.892 1.919 1 -1 Ne 10 11 21 -0.660 0.159 1 -3 Na 11 12 23 2.220 2.799 1 0 Mg 12 13 25 -0.860 0.159 1 -3 Al 13 14 27 3.640 3.678 1 0 Si 14 15 29 -0.563 0.159 1 -3 P 15 16 31 1.133 1.039 1 -2 S 16 17 33 0.640 0.159 1 -3 Cl 17 18 35 0.820 1.039 1 -2 Cl 17 20 37 0.680 1.039 1 -2 K 19 20 39 0.390 1.039 1 -2 K 19 22 41 0.210 1.039 1 -2 Ca 20 23 43 -1.320 1.919 1 -1 Sc 21 24 45 4.765 5.678 1 2 Ti 22 25 47 -0.795 0.159 1 -3 Ti 22 27 49 -1.100 1.919 1 -1 Cr 24 29 53 -0.475 0.159 1 -3 Mn 25 30 55 3.456 3.678 1 0 Fe 26 31 57 0.096 0.159 1 -3 Co 27 32 59 4.636 3.678 1 0 This table shows the mass loss in electron/positron pairs. BOOK DIFFERENCES A Z Calc WT Book WT DIFF WT Per Z Neutrino Per Z ELEC -1 0 0.000000 0.000549 0.000549 0.000000 0.65 100.00 NEU 0 1 1.007825 1.008665 0.000840 0.000840 1.00 1.00 H 1 1 1.007825 1.007825 0.000000 0.000000 0.00 0.00 1 2 2.015650 2.014105 -0.001544 -0.000772 -1.84 -0.92 1 3 3.023474 3.016049 -0.007425 -0.002475 -8.84 -2.95 HE 2 3 3.023474 3.016030 -0.007444 -0.002481 -8.86 -2.95 2 4 4.031299 4.002604 -0.028695 -0.007174 -34.17 -8.54 2 5 5.039124 5.012296 -0.026828 -0.005366 -31.94 -6.39 2 6 6.046948 6.018900 -0.028049 -0.004675 -33.40 -5.57 LI 3 5 5.039124 5.012541 -0.026583 -0.005317 -31.65 -6.33 3 6 6.046948 6.015126 -0.031822 -0.005304 -37.89 -6.32 3 7 7.054773 7.016005 -0.038768 -0.005538 -46.16 -6.59 3 8 8.062598 8.022488 -0.040111 -0.005014 -47.76 -5.97 3 9 9.070423 9.027300 -0.043123 -0.004791 -51.35 -5.71 BE 4 6 6.046948 6.019780 -0.027168 -0.004528 -32.35 -5.39 4 7 7.054773 7.016931 -0.037842 -0.005406 -45.06 -6.44 4 8 8.062598 8.005308 -0.057290 -0.007161 -68.21 -8.53 4 9 9.070423 9.012186 -0.058237 -0.006471 -69.34 -7.70 4 10 10.078248 10.013535 -0.064713 -0.006471 -77.05 -7.71 B 5 8 8.062598 8.024612 -0.037986 -0.004748 -45.23 -5.65 5 9 9.070423 9.013335 -0.057088 -0.006343 -67.97 -7.55 5 10 10.078248 10.012939 -0.065309 -0.006531 -77.76 -7.78 5 11 11.086073 11.009305 -0.076768 -0.006979 -91.41 -8.31 5 12 12.093897 12.014353 -0.079544 -0.006629 -94.71 -7.89 C 6 10 10.078248 10.016830 -0.061418 -0.006142 -73.13 -7.31 6 11 11.086073 11.011433 -0.074640 -0.006785 -88.87 -8.08 6 12 12.093897 12.000000 -0.093897 -0.007825 -111.80 -9.32 6 13 13.101722 13.003354 -0.098368 -0.007567 -117.12 -9.01 6 14 14.109547 14.003242 -0.106305 -0.007593 -126.58 -9.04 6 15 15.117372 15.010600 -0.106771 -0.007118 -127.13 -8.48 N 7 12 12.093897 12.018709 -0.075188 -0.006266 -89.52 -7.46 7 13 13.101722 13.005739 -0.095983 -0.007383 -114.28 -8.79 7 14 14.109547 14.003074 -0.106473 -0.007605 -126.78 -9.06 7 15 15.117372 15.000108 -0.117264 -0.007818 -139.62 -9.31 7 16 16.125196 16.006088 -0.119108 -0.007444 -141.82 -8.86 7 17 17.133020 17.008450 -0.124571 -0.007328 -148.32 -8.72 O 8 14 14.109547 14.008597 -0.100949 -0.007211 -120.20 -8.59 8 15 15.117372 15.003072 -0.114300 -0.007620 -136.09 -9.07 8 16 16.125196 15.994915 -0.130281 -0.008143 -155.12 -9.70 8 17 17.133020 16.999132 -0.133888 -0.007876 -159.42 -9.38 8 18 18.140846 17.999161 -0.141685 -0.007871 -168.70 -9.37 8 19 19.148670 19.003557 -0.145113 -0.007638 -172.78 -9.09 8 20 20.156496 20.004070 -0.152426 -0.007621 -181.49 -9.07 F 9 17 17.133020 17.002098 -0.130922 -0.007701 -155.89 -9.17
-
Swansont said: Any of them. Dipole and higher-order multipole moments are known and measured for nuclei. You have to show that your model matches them. Reply: That is an excellent point. That needs to be answered but also provides a means of determining the structure of the models. Much better than just picking the pretty ones. How to put the Tinkertoys together to match the dipoles is not immediately obvious. i will head back into my cave and stew about it. Thanks again for sharing your very extensive knowledge of the subject.
-
swansont Posted 22 August 2013 -11:00 AM Swanson said: Again, your models imply electric dipole moments which are not observed. Reply: The charges are pretty evenly distributed so there shouldn't be significant difference in the charge from one side of the nucleus to the other. The instable isotopes may have a slight electric dipole moment. Which model are you referring to? Some light nuclei models are below. (If the picture is visable) ***************************** Swansont said: The nuclei are not shaped like this. Reply: Tell me the shape of a nucleus and I will try to produce a model to match. ***************************** Swansont said: The binding energy of H-2 is 2.224 Me\/. The energy of two e-e+ pairs is 2.044 Iv1e\/. You're off by 9%. Your other numbers seem to be ad-hoc. In any event, you can't have 114.29 pairs of particles. There has to be an integral number. Reply: The reason the numbers don't come out even is that there is more than one way to overlap the protons resulting is a different number of electron/positron pairs missing. Somewhat similar to the way isotopes of elements work. The 2.224 Mev binding energy is an averaged number. ***************************** Swansont said: In any event, this is not the binding energy. I'm talking about the configuration energy of all of these charges — there is an electrostatic potential energy for this configuration. What is it? Reply: That is a very difficult calculation. You have to know the position of each electron and positron in each of the protons then do calculation for each one. However, it may be possible to get an approximation to show that the total comes out negative. Since some of the protons in the nucleus share electron/proton pairs so the multiple interactions between electrons and positrons make a stronger bond than the bond between 1 electron and 1 proton. For instance, the bond between 2 pairs is about 1.4 times as great as 1 pair. + - + as apposed to - + - ***************************** Swansont said: If you are going to claim a proton is made up of 2399 particles. I must ask for evidence to support the claim. Are you noticing a pattern yet? A claim always instigates a request for evidence. Maybe you could start anticipating this. Reply: The fact that the difference in mass between the neutron and the hydrogen atom is divisible into both of them to 7 digits of accuracy is strong evidence of a building block. Since both electrons and positrons can be emitted by decay that should mean that there are electrons and positrons in the nucleus. Even if they are "created on expulsion" this model can accommodate that because all matter consists of the same positive and negative vector like entities. ***************************** Another try at the picture of the models. Still another try with a picture from WORD. Sorry still no picture. Eureka ! The picture posted.
-
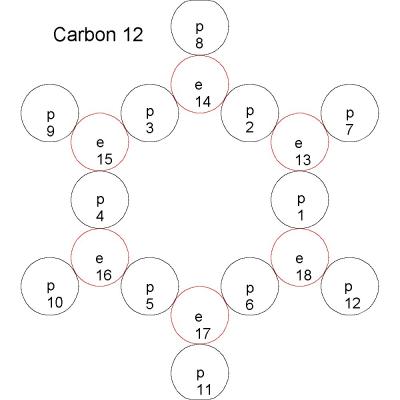
Swansont said: We also don't accept things as correct just because there is no evidence against it. You need to have predictions that can be tested, and your model has to match up with what we've already observed. I pointed out several problems in my last post. Reply: I apologize for missing your post. ***************************************** Swansont said: So you have an unstable equilibrium. What happens when the system is perturbed? It should collapse in on itself. Reply: This arrangement of electrons is just to demonstrate that electrons can hold more protons together than the number of electrons. To get strong enough forces to build satisfactory models it is necessary to consider the building blocks of protons, namely electrons and positrons. The proton could be modeled in the following manner as one possibility. Someone said the scattering pattern of the proton is like 3 point masses. May be 3 humps would do instead of point masses. The +'s are positrons and the -'s are electrons. The three hump model PROTON A side view is something like this: Sequence Layer Count Charge Net 1 * 1 1 +1 +1 2 * * * 2 4 +2 +3 3 * * * * * 3 13 +1 +4 4 * * * * * * * 4 22 -2 +2 5 * * * * * * * * 5 37 +1 +3 6 * * * * * * * * * 6 52 -2 +1 7 * * * * * * * * * * 7 73 +1 +2 8 * * * * * * * * * * * 8 94 -2 +0 9 * * * * * * * * * * * 8 94 +2 +2 10 * * * * * * * * * * * 8 94 -2 0 11 * * * * * * * * * * * 7 73 +1 +1 12 * * * * * * * * * * 6 52 -2 -1 13 * * * * * * * * * 5 37 +1 0 14 * * * * * * * 4 22 -2 -2 15 * * * * * 3 13 +1 -1 16 * * * * * 3 13 -1 -2 17 * * * * * * * 4 22 +2 0 18 * * * * * * * * * 5 37 -1 -1 19 * * * * * * * * * * * 6 52 +2 +1 20 * * * * * * * * * * * * 7 73 -1 +0 21 * * * * * * * * * * * * 7 73 +1 +1 22 * * * * * * * * * * * * * 8 94 -2 -1 23 * * * * * * * * * * * * * 8 94 +2 +1 24 * * * * * * * * * * * * * * 9 121 -1 +0 25 * * * * * * * * * * * * * 8 94 +2 +2 26 * * * * * * * * * * * * * 8 94 -2 0 27 * * * * * * * * * * * * 7 73 +1 +1 28 * * * * * * * * * * * * 7 73 -1 0 29 * * * * * * * * * * * 6 52 +2 +2 30 * * * * * * * * * * 5 37 -1 +1 31 * * * * * * * 4 22 +2 +3 32 * * * * * 3 13 -1 +2 33 * * * * * 3 13 +1 +3 34 * * * * * * * 4 22 -2 +1 35 * * * * * * * * 5 37 +1 +2 36 * * * * * * * * * 6 52 -2 0 37 * * * * * * * * * * 7 73 +1 +1 38 * * * * * * * * * * * 8 94 -2 -1 39 * * * * * * * * * * * 8 94 +2 +1 40 * * * * * * * * * * * 8 94 -2 -1 41 * * * * * * * * * * * 7 73 +1 0 42 * * * * * * * * * * 6 52 -2 -2 43 * * * * * * * * * 5 37 +1 -1 44 * * * * * * * 4 22 -2 -3 45 * * * * * 3 13 +1 -2 46 * * * 2 4 +2 +0 47 * 1 1 +1 +1 The net charge is +1. I contains 2399 particles. It is made up 47 layers of particles arranged in hexagons. There are 9 different size layers. The particles adjacent to each other are of opposite charge. The particle in the center of the proton is negative. The particle on each end is positive. The layers that make up the structure are as follows. Layers 4 through 8 may have the charges reversed. Layer size 1 Number of particles is 1. Charge is +1. + Layer size 2 Number of particles is 4. Charge is +2. + - + + Layer size 3 Number of particles is 13. Charge is +1. - + + - - + + + - - - + + Layer size 4 Number of particles is 22. Charge is +2 or -2 - - - + + + + - - - - + + + - - - + + - - + Layer size 5 Number of particles is 37. Charge is +1 or -1. Charge is +2 Charge is -2 + + - - - - - + + + + + + - - - - - - - + + + + + + + + - - - - - - - - - + + + + + + + + + + - - - - - - - - - + + + + + + + + - - - - - - - + + + The way to get a strong bond is for the protons to share electrons and positrons. That explains why bigger nuclei have less mass than a multiple of hydrogen. The mass loss on of about 10 electrons and 10 positrons matches the heavier atoms. The mass loss for hydrogen 2 is 2 electrons and 2 positrons or 1 pair per proton.. The loss for hydrogen 3 is 9 electrons and 9 positrons or 3 pairs per proton.. The loss for helium 4 is 34 electrons and 34 positrons or 8.5 pairs per proton. The loss for copper is 670 electrons and 670 positrons or 10.65 pairs per proton. The loss for uranium 238 is 2157 electrons and 2157 positrons or 9.06 pairs per proton. The reason the numbers don't come out even is, somewhat like isotopes of atoms, is that there can be more than one way of connecting the electrons and positrons resulting in a different number of lost electrons and positrons. ************************************* Swansont said: And, the next step: calculate the energy of the system, and compare it to the known binding energy of C-12. Reply: The binding energy should match the mass loss. The mass loss of carbon 12 is .093897 AMU compared to the hydrogen 1 atom which equates to 114.28 pairs of electrons and positrons. ************************************* Swansont said: Also, what if you lack the symmetry you have here? What of Li-7, or C-13? What of Deuterium? Shouldn't there be a nuclear electric dipole moment in deuterium, according to your model? Reply: With the strong bond of protons sharing electrons and positrons 3D models can be construted. Lithium 6 Showing the 6 protons 1 1 1 2 3 2 3 2 444 3 5 6 5 6 5 6 For lithium 6 a flat model looks pretty good. Lithium 7 1 1 7 1 2 3 7 2 3 2 444 3 7 5 6 5 6 5 6 Sorry, the 3D model for lithium 7 is hard to follow. I haven't figured out how to put pictures in a post. Carbon 12 and Carbon 13 are built as 3D in a similar manner but I have a problem drawing them here.
-
That is sensible and is probably the strongest objection to this model as the discrete orbits could be caused by the rotating magnetic field of the nucleus. However, that may not mean it is impossible that an electron in orbit that is the size of an atom to have some mechanism that defeats the radiation. The photon is so much longer than the atom's diameter that the electron can complete one or more cycles before the the photon is gone. If the photon is leaving in the east direction on one side it should be leaving in the west direction on the opposite side. Nether photon will have completely left the electron during a cycle. A physical situation that has a little similarlarity is: Attach a bucket to a horizonal wheel. Put a coil of rope in the bucket with a couple of feet hanging over the side. Spinning the wheel will cause the rope to be pulled out of the bucket and go off into space. Now attach a vertical pole to the bucket so it extends a bit above the bucket.. Spinning the wheel will cause the rope to wrap around the pole and not fly off into space. Since we do not fully understand the mechanism of an electron firing a photon, it may be a bit of a stretch to say that it is not possible for an electron to orbit a nucleus without radiating.
-
f is the scalar force between the test proton and the current particle. nfx,nfy is the vector sum as we go dow the list. at the bottom or the list nfx,nfy is the vector of the net force on the test proton.
-
Swansont said: And? What is the result of your calculation? Am I to understand you have done the calculation for your ring shape, and gotten a net attraction for all protons? Reply: Here are the forces one proton from all the other particles. The arithmetic may not be correct but the values from my program are here. If you wish, I can tell the program to choose other particles or other nuclei. Lithium 6 p6 p2 e7 p5 e8 p1 e9 p3 p6 Net force vector (nfx,nfy) on proton number 4 Atomic number is 3 Proton 4 at x= 1.000 y= 1.732 Plus x is repulsive, minus x is attractive Number x y d f nfx nfy q 1 1.000 0.000 1.732 0.333 -0.000 0.333 1 2 -0.500 0.866 1.732 0.333 0.289 0.500 1 3 -0.500 -0.866 3.000 0.111 0.344 0.596 1 5 -2.000 -0.000 3.464 0.083 0.416 0.638 1 6 1.000 -1.732 3.464 0.083 0.416 0.721 1 7 0.500 0.866 1.000 1.000 -0.084 -0.145-1 8 -1.000 -0.000 2.646 0.143 -0.192 -0.238-1 9 0.500 -0.866 2.646 0.143 -0.219 -0.379-1 Attractive force electron to proton -1.000 Total repulsive force on proton -0.437 ----------------------------------------------------- Net force vector (nfx,nfy) on proton number 7 Atomic number is 6 Proton 7 at x= 1.500 y= 0.866 Plus x is repulsive, minus x is attractive Number x y d f nfx nfy q 1 1.000 0.000 1.000 1.000 0.500 0.866 1 2 0.500 0.866 1.000 1.000 1.500 0.866 1 3 -0.500 0.866 2.000 0.250 1.750 0.866 1 4 -1.000 -0.000 2.646 0.143 1.885 0.913 1 5 -0.500 -0.866 2.646 0.143 1.993 1.006 1 6 0.500 -0.866 2.000 0.250 2.118 1.223 1 8 -0.000 1.732 1.732 0.333 2.407 1.056 1 9 -1.500 0.866 3.000 0.111 2.518 1.056 1 10 -1.500 -0.866 3.464 0.083 2.590 1.098 1 11 0.000 -1.732 3.000 0.111 2.646 1.194 1 12 1.500 -0.866 1.732 0.333 2.646 1.527 1 13 1.000 0.577 0.577 3.000 0.047 0.027-1 14 -0.000 1.155 1.528 0.429 -0.374 0.108-1 15 -1.000 0.577 2.517 0.158 -0.530 0.090-1 16 -1.000 -0.577 2.887 0.120 -0.634 0.030-1 17 0.000 -1.155 2.517 0.158 -0.728 -0.097-1 18 1.000 -0.577 1.527 0.429 -0.869 -0.502-1 Attractive force electron to proton -3.000 Total repulsive force on proton -1.003 -------------------------------------------------------- Uranium 270 O Net force vector (nfx,nfy) on proton number 93 Atomic number is 92 Proton 93 at x= 1.058 y= 0.036 Plus x is repulsive, minus x is attractive Number x y d f nfx nfy q 1 1.000 0.000 0.068 214.487 181.980 113.525 1 2 0.998 0.068 0.068 214.487 371.283 12.684 1 3 0.991 0.136 0.121 68.835 409.688 -44.442 1 91 0.991 -0.136 0.185 29.234 489.730 -41.700 1 92 0.998 -0.068 0.121 68.850 524.158 17.925 1 94 1.053 0.108 0.072 191.408 537.220 -173.037 1 274 1.004 -0.173 0.216 21.424 -215.954 166.582-1 275 1.014 -0.104 0.147 46.198 -229.816 122.512-1 276 1.019 -0.035 0.081 151.935 -303.600 -10.304-1 Attractive force electron to proton -643.469 Total repulsive force on proton -303.775
-
Swansont said: You need math to back this up. How exactly does a nucleus with net positive charge stay together with only an electrostatic force? Reply: To test the ability of the electron's force, calculate the net force vector on one of the most vunerable protons. The protons furthest from the origin are most vunerable. Or if you wish test any proton. The test proton has the greatest x value and is at coordinates (a,b). q is the charge of a particle. (1 or -1) d is the distance from the test particle. d = sqr((a-x)^2 + (b-y)^2) The force vector on the test particle from another particle is: q(a-x)i q(b-y)j F = ------- + ------- d^3 d^3 The sum of all the force vectors is: gi + hj The net force, f is; f = sqr(g^2 + h^2) The attraction or repulsion is dependent on the sign of g. Place the center of the nucleus at (0,0) of the coordinate system, Then rotate the nucleus until one of the protons closest to the origin is on the positive x axis. The proton with the smallest angle from it in the counter clockwise direction is the test proton. If the net force vector on the test proton is positive the proton will be expelled, if it is negative the proton will be held in place. The calculation of the force vector between two particles is straight forward. Then you have to add up all the force vectors to find the net force and the direction of the force. An example of of a way electrons could hold protons together. In this case we would have p1 on the x axis and p7 as the test proton. The x axis goes through p4 and p1, the y axis goes through p8 and p11.
-
Swansont said: This explains nothing. Neutrino oscillations has an actual meaning in physics, and it doesn't apply here, as it is a change of one type of neutrino to another. The problem with your model is that there is no neutrino. How do you account for the appearance of the neutrino in decay? Where do the positrons come from in beta+ decay? Reply: The neutrino consists of an electron and a positron. The protons are full of electrons and positrons. ************************************************************************************************************************************************* Swansont said: Then go find that reason. You can't just hand-wave the problem away by saying there must be a reason. Reply: The reason for the distinct orbits of electrons about the nucleus is that the nucleus has a rotating magnetic field. The 2 lowest energy electrons orbit the nucleus in sync with the rotation of the magnetic field. The rotation time of the magnetic field is half the time of orbit of one of the 2 shell 1 electrons. In shell 2 with 8 electrons, an electron takes 12.5 times as long to complete 1 orbit as and electron in shell 1. That means when a shell 2 electron reaches its perigee the 2 shell 1 electrons will be exactly reversed in their positions. Each of the 8 shell 2 electrons arrives at its perigee when the 2 shell 1 electrons are in their reversed positions during the 200 cycles of a shell 1 electron. The rest of the shells have different numbers but behave in a similar manner. **************************************************************************************************************** Swansont said: Appealing to the stupidity of other scientists is probably not the best argument to use, but it doesn't matter because you don't actually have a model that uses a rotating magnetic field to explain anything. You can't just say "rotating magnetic field" as some sort of magic invocation. How does this actually solve anything? There is nothing stupid about not finding a solution because of missing or unknown data. The discription of the effect of a rotating magnetic field is above and also in the summary. ********************************************************************************************************************************************* SUMMARY OF THE MODEL The Red Shift of light is caused by electrons changing orbits about the nucleus. The reason for the distinct orbits of electrons about the nucleus is that the nucleus has a rotating magnetic field. The 2 lowest energy electrons orbit the nucleus in sync with the rotation of the magnetic field. The rotation time of the magnetic field is half the time of orbit of one of the 2 shell 1 electrons. In shell 2 with 8 electrons, an electron takes 12.5 times as long to complete 1 orbit as and electron in shell 1. That means when a shell 2 electron reaches its perigee the 2 shell 1 electrons will be exactly reversed in their positions. Each of the 8 shell 2 electrons arrives at its perigee when the 2 shell 1 electrons are in their reversed positions during the 200 cycles of a shell 1 electron. The rest of the shells have different numbers but behave in a similar manner. The reason clocks slow in gravity is that the gravity affects the orbit of the electrons about the nucleus. The reason clocks slow at high velocities is that the limitation of the speed of light causes the path of the electron to change. The reason particles and photons are limited to the speed of light is that all particles and photons consist of small entities that can be represented as positive and negative vectors which always travel at the speed of light. That implies that force on them can only be effective perpendicular to the direction of travel. There is no Nuclear Binding Force. The protons in a nucleus are held together by electrons. The neutron consists of 1 proton, 2 electrons and a positron. The proton and the neutron consist of electrons and positrons. The neutrino consists of an electron and a positron. Light slows in a medium because the particles cause the photon to change directions to go around them. The reason that an electron has an electromagnetic charge greater than a charge of 1 rotating on the surface of an electron is that there are multiple positive and negative charged entities in the electron. The reason there is no dispersion of the colors when light passes the sun is that the particles around the sun are far enough apart that the photons don't always hit feet first as they do with a prism.
-
Swansont said: The claim appears to be that the 6 neutrons are really 6 protons + 6 electrons, but now we have a problem with e.g. C-14 which would have 14 protons and 8 electrons, and undergoes beta decay. You have the electron emitted from the nucleus, but now have to explain where the antineutrino came from. Worse, you have neutron-deficient nuclei which undergo beta-plus decay. Reply: The various 3d configurations would have some stronger and some weaker bonds. With 2D models the "pretty" ones seem to coorespond to the more stable isotopes. The cause of radiation has been consideted to be neutino collisions with nuclei. The configurations that are not nice and neat would be more suceptable to ejection of particles. In the early 1900's, when attempts were being made to come up with a physical model, the reason they gave up was for the same 2 reasons you gave, the electrons should radiate and they had no explanation as to why the electron orbits were discrete. I am sure they would have or did come up with a reason that the electon in orbit didn't radiate since the photon is so much bigger than the atom that a photon leaving could be offset by one in the opposite direction. Not a smoking gun. The reason they couldn't come up with an explanation for the discrete orbits is that they had no clue that there could be a rotationg magnetic field around the nuculeus. The Heisenberg Uncertainty Principle is usefull but doesn't preclude there being something real that we are being uncertain about.
-
Agreed. The ring structure just demonstrates that electrons can bind multiple protons and it is easier to visualize. There is something else that had to be resolved to make more satisfactory models. Three protons attached to an electron is insufficient to build good 3D models and 4 protons attached to an electron is too weak of a bond. The solution is indicated by the loss of nominal mass as we go up the atomic numbers and the fact that the difference between the mass of the neutron and the hydrogen atom is evenly divisable to 7 digits of accuracy into both indicating a building block which could consist of an electron and a positron.. That would imply that the joining of an eledtron and a positron could resust in some mass loss as apposed to annialating each other. The reduced mass of bigger nuclei can result form sharing the building blocks resulting in a stronger bond between protons. That allows compact models to be constructed. Another problem that had to be solved. The radius of an electron used to be considered more than twice the radius of the proton. Now the electron is thought bo be smaller than that. That helps to let the protons overlap.
-
That is the nucleus, not the atom.
-
Reply: g is the component of the net force vector along the x axis. If g is positive the proton will be expelled. if negative ithe proton will stay in place. Correction to the reply to Mellinia Reply: Place the center of the nucleus at (0,0) of the coordinate system, --------------------------------------------------------------------------------------------------------- Wrong Then rotate the nucleus until one of the protons furthest from the origin is on the positive x axis. That proton is the test proton. Should read: Then rotate the nucleus until one of the protons closest to the origin is on the positive x axis. The proton with the smallest angle from it in the counter clockwise direction is the test proton. --------------------------------------------------------------------------------------------------------------- If the net force vector on the test proton is positive the proton will be expelled, if it is negative the proton will be held in place. The calculation of the force vector between two particles is straight forward. Then you have to add up all the force vectors to find the net force and the direction of the force.
-
x is the distance from the origin of a 2 dimentional coordiate system. I really don't understand why I am getting so many complaints about the arithmetic. It is just high school math. I am not complaining, I appreciate any comment.
-
Strange said: Well, if you can't present the math ... I am not going to try and reverse engineer your code The test proton has the greatest x value and is at coordinates (a,b). q is the charge of a particle. (1 or -1) d is the distance from the test particle. d = sqr((a-x)^2 + (b-y)^2) The force vector on the test particle from another particle is: q(a-x)i q(b-y)j F = ------- + ------- d^3 d^3 The sum of all the force vectors is: gi + hj The net force, f is; f = sqr(g^2 + h^2) The attraction or repulsion is dependent on the sign of g. Swansont said: Further, the Bohr atom doesn't have elliptical orbits! They are circular (one part of its wrongness, in many ways, for many reasons). Any analysis past this point of not having a valid model is a wasted effort. Reply: This model is not the Bohr Model, the Bohr-Summerfield Model, the Bohr-Kramers-Slater Model nor the Rydenberg Model. The biggest similarity is that attempts are being made to describe the actual reality that underlies the probability waves. These people and others made a lot of progress accounting for split lines, fine lines and other relationships. If they had known what we now know that it is possible for nuclei to have rotating magnetic fields, which allows distinct orbits, things might have gone in a different direction. Elliptical orbits were considered long ago. The only substantial objection to this model is your point that an electron on a curved path should radiate. Not only is the momentum changing but the kinetic energy of the electron would vary in the orbit. One possible explanation is that the photon is so much bigger than a atom that an electron could make one or more complete circuits while a photon was coming or going. That could allow the kinetic energy the photon leaving in one part of the orbit to be reacquired on the opposite side. As for the momentum, the photon should leave in opposite directions on opposite sides of the ellipse, which might cause them to cancel each other.
-
Strange said: And what does "but a better number is .00083985429 AMU" mean? Reply: The number .00083985428 divides the mass of both the proton and neutron evenly to 7 significant digits implying a "building block" for both of them. Do you have a better explanation for that or do you just prefer the 1 in 10 million chance that it is pure coincidence. Also, it pure coincidence that the arithmetic of an electron orbiting a nucleus can show the speed of galaxies. In addition, it is pure coincidence that the synchronizing of the movement of electrons in the Bohr atom shows that in elliptical orbits the electrons will take turns approaching the nucleus. ***************************************************** Strange said: Pretty pictures but without the math, I don't believe it works. Reply: I wrote a couple of programs to show the mathematics involved just for you. One does the positioning of the electrons and protons and the other does the force calculations. A picture of the model of the nucleus is followed by the results of the calculations. The programs are at the end of the post. Net force vector (nfx,nfy) on proton number 4 Atomic number is 3 Proton 4 at x= 1.000 y= 1.732 Plus x is repulsive, minus x is attractive Number x y d f nfx nfy q 1 1.000 0.000 1.732 0.333 -0.000 0.333 1 2 -0.500 0.866 1.732 0.333 0.289 0.500 1 3 -0.500 -0.866 3.000 0.111 0.344 0.596 1 5 -2.000 -0.000 3.464 0.083 0.416 0.638 1 6 1.000 -1.732 3.464 0.083 0.416 0.721 1 7 0.500 0.866 1.000 1.000 -0.084 -0.145-1 8 -1.000 -0.000 2.646 0.143 -0.192 -0.238-1 9 0.500 -0.866 2.646 0.143 -0.219 -0.379-1 Attractive force electron to proton -1.000 Total repulsive force on proton -0.437 Net force vector (nfx,nfy) on proton number 7 Atomic number is 6 Proton 7 at x= 1.500 y= 0.866 Plus x is repulsive, minus x is attractive Number x y d f nfx nfy q 1 1.000 0.000 1.000 1.000 0.500 0.866 1 2 0.500 0.866 1.000 1.000 1.500 0.866 1 3 -0.500 0.866 2.000 0.250 1.750 0.866 1 4 -1.000 -0.000 2.646 0.143 1.885 0.913 1 5 -0.500 -0.866 2.646 0.143 1.993 1.006 1 6 0.500 -0.866 2.000 0.250 2.118 1.223 1 8 -0.000 1.732 1.732 0.333 2.407 1.056 1 9 -1.500 0.866 3.000 0.111 2.518 1.056 1 10 -1.500 -0.866 3.464 0.083 2.590 1.098 1 11 0.000 -1.732 3.000 0.111 2.646 1.194 1 12 1.500 -0.866 1.732 0.333 2.646 1.527 1 13 1.000 0.577 0.577 3.000 0.047 0.027-1 14 -0.000 1.155 1.528 0.429 -0.374 0.108-1 15 -1.000 0.577 2.517 0.158 -0.530 0.090-1 16 -1.000 -0.577 2.887 0.120 -0.634 0.030-1 17 0.000 -1.155 2.517 0.158 -0.728 -0.097-1 18 1.000 -0.577 1.527 0.429 -0.869 -0.502-1 Attractive force electron to proton -3.000 Total repulsive force on proton -1.003 Net force vector (nfx,nfy) on proton number 93 Atomic number is 92 Proton 93 at x= 1.058 y= 0.036 Plus x is repulsive, minus x is attractive Number x y d f nfx nfy q 1 1.000 0.000 0.068 214.487 181.980 113.525 1 2 0.998 0.068 0.068 214.487 371.283 12.684 1 3 0.991 0.136 0.121 68.835 409.688 -44.442 1 91 0.991 -0.136 0.185 29.234 489.730 -41.700 1 92 0.998 -0.068 0.121 68.850 524.158 17.925 1 94 1.053 0.108 0.072 191.408 537.220 -173.037 1 274 1.004 -0.173 0.216 21.424 -215.954 166.582-1 275 1.014 -0.104 0.147 46.198 -229.816 122.512-1 276 1.019 -0.035 0.081 151.935 -303.600 -10.304-1 Attractive force electron to proton -643.469 Total repulsive force on proton -303.775 ***************************************************** Programs ' calcbnuc.bas defdbl a-z ' Calc positions of electrons and protons in a nucleus ' Circular models only ' ' Input Atomic number for different atoms ' Formlas ' r=1 Radius of inner circle of atom ' x=r*cos(theta)' Polar to orthogonal location of proton or electron ' y=r*sin(theta) ' Trig functions ' An example of circular models ' beryllium 8 ' p p p ' e e ' p p ' e e ' p p p ' ' Numbered ' ' 6 2 5 ' 10 9 ' 3 1 ' 11 12 ' 7 4 8 ' open "calcnuc.txt" for output as#8 open "junk" for output as#9 print"What is the atomic number of the atom?" input z z=int(z) atomno=z if z<3 then print" The number can't be less than 3" input a$ goto c9999' Outta here end if howmany=atomno' Number of protons in inner circle pi=3.1416' Pi twopi=pi*2 r=1' Set radius of inner circle to 1 anglebig=twopi/howmany' Angle between protons in inner circle angsmall=anglebig/2' Angle between every particle ri=1' ri is radius of inner circle of protons = 1 ' Calculate radiuso, radius of outer circle of protons x1=1' Coordinates of proton number 1 y1=0 x2=r*cos(theta+anglebig)' Coordinates of proton number 2 y2=r*sin(theta+anglebig) xc=r*cos(theta+angsmall)' Coordinates of point on circle to proton 2 yc=r*sin(theta+angsmall) xm=(x1+x2)/2' Mid point between protons 1 and 2 ym=(y1+y2)/2 distp1p2=sqr((x1-x2)*(x1-x2)+(y1-y2)*(y1-y2))' Distance p1 to p2 distmpo=distp1p2*.866'Dist mid to outer.Equilateral triangle rp=distmpo/3' Radius of particles. Diagonal is 2/3 of angle bisector distcl=sqr((xc-xm)*(xc-xm)+(yc-ym)*(yc-ym))'Distance circle vs line ro=1+distmpo-distcl' Radius of outer circle ' Calculate radiusm, radius of middle circle of electrons rm=ro-rp*2' Radius of middle circle theta=0' Angle to particle. A proton is on x axis print#9, print#9," Inner protons" print#9, for i=1 to howmany' Do the inner protons x=ri*cos(theta)' Convert from polar to rectangular cordinates y=ri*sin(theta) print#8,x;",";y;",";1;atomno;rp'x, y, sign and radius of particle print#9,x;",";y;",";1;atomno;rp'x, y, sign and radius of particle theta=theta+anglebig' Increment around circle next i print#9, print#9," Outer protons" print#9, theta=angsmall for i=1 to howmany' Do the outer protons x=ro*cos(theta)' Convert from polar to rectangular cordinates y=ro*sin(theta) print#8,x;",";y;",";1;atomno;rp'x, y, sign and radius of particle print#9,x;",";y;",";1;atomno;rp'x, y, sign and radius of particle theta=theta+anglebig' Increment around circle next i print#9, print#9," Electron" print#9, theta=angsmall for i=1 to howmany' Do the inner electrons x=rm*cos(theta)' Convert from polar to rectangular cordinates y=rm*sin(theta) print#8,x;",";y;",";-1;atomno;rp'x, y, sign and radius of particle print#9,x;",";y;",";-1;atomno;rp'x, y, sign and radius of particle theta=theta+anglebig' Increment around circle next i c9999:' Done ******************************************************************** ' calcrepu.bas ' Calculate the force on a vunerable proton in the nucleus open "calcnuc.txt" for input as#1 open "calcrepu.txt" for output as#8 dim xx(500)' The electrons and protons dim yy(500) dim charge(500) dim theta(500) ct=0' Count of particles c12: if eof(1) goto c19' Read in the electrons and protons ct=ct+1 input#1,xx(ct),yy(ct),charge(ct),atomno,radius goto C12 ' Calculate the net force on a proton c19: particle=ct/3+1' Choose a proton in the outer circle to test atomno=ct/3' Atomic number of atom print#8," Net force vector (nfx,nfy) on proton number"; print#8,particle print#8," Atomic number is";atomno; print#8," Proton"; print#8,particle; print#8,"at x="; print#8,using"##.###";xx(particle); print#8," y="; print#8,using"##.###";yy(particle)' print#8," Plus x is repulsive, minus x is attractive" print#8, Print#8,"Number x y d f nfx nfy q" gosub calcnet' Calculate net force vector. + is out, - is in. repulsef=sqr(nfx*nfx+nfy*nfy)' Calculate repulsive force if nfx<0 then repulsef=-repulsef' Negative if attractive print#8, print#8," Attractive force electron to proton ";using"#####.###";-maxforce print#8," Total repulsive force on proton ";using"#####.###";repulsef goto c8' Done '----------------------------------------------------- ' calcnet subroutine calcnet: x=xx(particle)' Remember which proton to test y=yy(particle) maxforce=0' Remember maximum e to p force nfx=0' Net force vector for the proton nfy=0 for i=1 to ct' Loop through all the particles if i=particle goto c25' Skip ourself if charge(i)=1 then dx=x-xx(i)' Distance vector from a proton dy=y-yy(i) end if if charge(i)<>1 then dx=xx(i)-x' Distance vector from an electron dy=yy(i)-y end if d=sqr(dx*dx + dy*dy)' Distance ux=dx/d' Unit vector uy=dy/d f=1/(d*d)' Force = inverse of distance squared if charge(i)=-1 then if maxforce<f then maxforce=f' Remember maximun e to p force end if fx=ux*f' Unit vector times force fy=uy*f nfx=nfx+fx' Add to net force nfy=nfy+fy print#8,using"###";i; print#8,using"###.###";xx(i);'Print the coordinates, distance and print#8,using"###.###";yy(i);' force of this proton print#8,using"###.###";d; print#8,using"#####.###";f; print#8,using"#####.###";nfx; print#8,using"#####.###";nfy; print#8,charge(i) c25: next i return c8: c9999: Sorry, pictures didn't take.
-
Strange said: Is that a reply to "And what does "but a better number is .00083985429 AMU" mean? If so, it is wrong. The sum of the electron and positron masses is 0.0010971598 (based on the Particle Data Group figures). Reply: One possible explaination is that puting a electron and a positon together could result is mass loss. That is rather than annihilating each other. Strange said: Pretty pictures but without the math, I don't believe it works. Reply: It is easy to see that the light elements could work. The heavier elements would not work in this cofiguration. By allowing the protons consisting of electrons and positons share some of them a stronger bond can be made. Also, that makes it easier to constuct 3D models.
-
Swansont said: No, that doesn't work. We know the orbital angular momentum of the S state is zero. In addition to (or separate from) the electron spin. Reply: I am not sure that applies to this model. If this model generates valid Red Shift values it may not matter. Swansont said: I take it that this non-response response means you have no justification. Reply: There are a great many excellent and very usefull algorithms that incorporate the Heisenberg concept but being able to calculate the probability of something doesn't mean that the real mechanism can't exist. I am still fretting about non radiating electron orbits. That seems to be a solid argument that has to be answered.
-
Strange said: Close, but no banana. Weight of H atom: 1.007825 Weight of Neutron: 1.008664916 Delta: 0.000839916 H / Delta: 1199.9116578323 N / Delta: 1200.9116578323 P / Delta: 1199.2585768099 Using your less accurate numbers gives a result even further from what you claim. And what does "but a better number is .00083985429 AMU" mean? It almost sounds like you have made it up to give the right answer... None of the ratios appear to be exact or have any physical meaning. Reply: That is the mass of a combined electron and positron. 1200 times that number gives the hydrogen mass the exact 7 digits of accuracy to the number you use plus 3 more digits. 1201 times the number gives the neutron mass matching your number to 7 digits of accuracy. The probability of pure coincidence is therefore 1 in 10 million. At least it is unlikely. ******** Strange said: Please show the math to support this. And show that it applies to atoms with large numbers of protons, such as lead and uranium. Reply: For an electron and 2 protons with the protons a distance of 1 from the electron the attraction between the electron and a proton is 1. The proton to proton repulsion is .25. So 1 > .25 For 3 protons the attraction between the electron and a proton is 1. For the repulsion of a proton from another proton the calculation is: Half the p to p distance is d/2 = sqr root of 3 divided by 2. The repulsive force is = 1/3. The force from the other 2 protons is 2/3 times cos(30). Net repulsive force on a proton is 1 / 1.732. So 1 > 1/1.732 The arithmetic for 4 protons is a little work. ******** The 2D models are easy to construct. Models can be constucted in 3D but it is necessary to use the proton consisting of electrons and positrons to build most of them. Greater attractive force is available.. Here are some heavier atoms. Helium 3 Helium 4 alpha particle p p p e e e (or) p p p p p e e p p p Z=2 A=3 Z=2 A=4 Z=2 A=4 Sodium 23 Sodium 24 p e p p p p p e e e p p p p p p e e p e e p p p p p e e epe p p p p p p p p e e e p e e p p p p p p e e p e e p p p p p p p p p p e e e p p p Z=11 A=23 Z=11 A=24 Platinum 198 P P P P E E E E P P P P P P P P P P P P E E E E E E P P P P P P E E E E E E P P P P P P P P P P P P P P P P E E E E E E E E P P P P P P P P P P E E E E E E E E E E P P P P P P P P P P P P P P P P P P P P E E E E E E E E E P P P P P P P P P E E E E E E E E E P P P P P P P P P P P P P P P P P P E E E E E E E E P P P P P P P P E E E E E E E E P P P P P P P P P P P P P P P P P P E E E E E E E E E P P P P P P P P P E E E E E E E E E P P P P P P P P P P P P P P P P P P P P E E E E E E E E E E P P P P P P P P P P E E E E E E E E P P P P P P P P P P P P P P P P E E E E E E P P P P P P E E E E E E P P P P P P P P P P P P E E E E P P P P Z=78 A=198 ******** Strange said: Here's one: you're wrong. Reply: If you can't give a reason just invoke Authority. I really do appreciate your comments.
-
Klaynos said: Nuclear physics works. So you are again wrong. Sorry. Reply: The equation named Roulette did a good job of predicting the motion of the sun and planets around the earth. Did that really prove that the sun orbits the earth? An equation can be made for any goofy concept and a goofy concept can be made for any equation. For example: d=vt distance = velocity times time. Postulate: The Mouse God is always in the same absolute location in space. Collary: When the Mouse God runs the universe moves at velocity, v. Consquence: A to find the absolute velocity of a human walking you must add the velocity of the Mouse God to the velocity of the human in ratty space. ------------------------------------------------- Strange said: I can't imagine why. Reply: You certainly understand my problem. ******** Strange said: I don't think so. Using the values from the Particle Data Group:Neutron mass: 1.008664916 Proton mass: 1.0072764668 Difference (delta): 0.0013884492 Proton/delta: 725.4687283608 Not exactly 'evenly divisible". But maybe you are thinking that looks close to the mass of an electron? Nope. It is 1.3224485911 times the mass of the electron. Reply: I goofed big time. It is the hydrogen atom not the proton that should be compared with the neutron.. See if I did it right this time. The weight of the hydrogen atom is 1.007825 Atom Mass Units (938 mev) and the neutron is 1.008665 AMU (939 mev). The difference i .000840 AMU (.789 mev) but a better number is .00083985429 AMU. If you multiply 1200 times .00083985429 you get 1.007825148, the weight of the hydrogen atom and 1201 times .00083985429 is 1.00866500229, the weight of the neutron. ******** Strange said: The trouble is, you still have a net positive charge in the nucleus. So you need to explain how the protons stay together given the electrostatic repulsion. Reply: If you put 2 protons on opposite sides of and electron the net charge is +1. However, the attraction between the electron and a proton is much greater than the repulsion of the protons. Same is true if you put 3 protons around and electron. The net charge is +2 but it will implode, not explode. Even 4 protons will implode but just barely. Over 20 years ago one of my judo buddies forced a friend of his who had a PHD in nuclear physics to talk to me. I described this and he said that he had never heard it or thought of it. ---------------------------------------------------------- Swansont Said: You're jumping the gun (in many ways). A semi-classical model with elliptical orbits would have to be tested first, before you can apply it and claim it to be responsible for a redshift. For starters, explain how you get an S state in hydrogen, which has zero orbital angular momentum, with this model. Reply: Let's rotate the electron enough to zero the rotational momentum. We could name it spin. ******** Swansont said: "Heisenberg Uncertainty Principle" Reply: I am somewhat in agreement with Bohr's "Don't tell God what to do" but I agree with Einstein's contention which is something like, "The cause and effect should not be thrown out. The lack of knowledge or ability to measure is behind the need for probability." . Tom has me snookered on justifying why orbiting electrons don't radiate . I really don't have a eon to figure it out. It would be nice if someone could give me a hint.
- 121 replies
-
-2
-
Swansont said: When I ask you to explain, I'm not looking for a repeat of what you just said. I'm asking how it's possible a magnetic field changes the kinetic energy of an electron, given (as I showed) that magnetic forces do no work. Reply: I was totally wrong on this. Tom's insisting made me rethink it and the problem was that I was only looking at one dimension of the force vector. Please excuse. I will go back into my cave and meditate on why the orbiting electron doesn't have to radiate. I should have it worked out in an eon or two. Before I head for the cave I would like to mention a few more pieces of this model. The Nuclear Binding Force does not exist. The nuclei consist of protons held together by electrons. The neutron consists of a proton, 2 electrons and a positron. The proton consists of electrons and positrons. The mass of the proton is evenly divisible by the difference of the mass of the proton and the neutron which implies a building block. All matter and radiation consists of quite small entities that can be represented by positive and negative vectors that always travel at the speed of light. This is the first time I have been able to get anyone knowledgeable to discuss any of this. Thanks to all of you and especially thanks to Tom. Ciao, Bob
- 121 replies
-
-1
-
Are these things wrong? The seem to be used in lots of equations. Swansont said: Their value does depend on the unit system, but that is not "arbitrary". An arbitrary constant takes on any value you want; you can choose it to be convenient. You can't choose H, G or c to be any value you want. Reply: Ok. I shouldn't say arbitrary. That is just the value the velocity of the electron must have at the apogee to give useful results. ************ Swansont Said: Explain how magnetic force does work to slow down a charged particle. F = qv X B, and Work is a dot product: dW = F.dx How can dx and F not be perpendicular? (which makes the dot product zero) Reply: The electron is accelerated in the Synchrotron by magneiic forces in a different part of the Synchrotron. At the corners the approach of the electron to the coil is equivalent to an increasing magnetic force which changes the kinetic energy of an electron. ******** Swansont said: The emission spectrum is not continuous. Not all colors are represented. Reply: Yes, this is a biggie. However, The distinct shells are possible with a rotating or pulsing magnetic field from the nucleus. The orbit of the electron would sync with the changing field. That means the second possible orbit would take twice as long to orbit as the smallest orbit. The trhird orbit would take 3 times as long, etc. An interesting observation about the circular Bohr atom is that in an atom with many electrons orbiting.every electron circling back to the same spot would find the electrons is the innermost shell in ether the same positions that they were or in reversed positions. With elliptical orbits that would be a nice way for electrons to take turns appoaching the nucleus. Further, the circular distance between any two adjacent electrons in any shell is the same. As I said earlier, It seemed very improbable tor the nucleus to have a rotating magnetic field but the Handbook of Nuclear Properties of 1997 mentions nuclei with magnetic forces having a rotational character. It appears possible that the elliptical Bohr atom could produce results consistant with the equations that are known to work.