UC
Senior Members-
Posts
547 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by UC
-
Random geocities sites are hardly valid evidence.
-
http://www.scienceforums.net/forum/showthread.php?t=23802 Nobody is going to help you unless you show that you've tried the problem first.
-

Getting rid of mast cells = cure for allergies?
UC replied to Green Xenon's topic in Microbiology and Immunology
I think we should require everyone who posts here to take 15 seconds and at the very least, go skim the wiki article on whatever they're posting about. http://en.wikipedia.org/wiki/Mast_cell Second sentence: "Although best known for their role in allergy and anaphylaxis, mast cells play an important protective role as well, being intimately involved in wound healing and defense against pathogens" -
You will quickly consume all the oxygen in the flask and not much SO2 will be generated. I suggest you give this sciencemadness.org thread a read and try that instead: http://www.sciencemadness.org/talk/viewthread.php?tid=2824
-
You've just experienced the magic of suckback, a common phenomenon. SO3 is extremely soluble in water, so there is no reason to submerge the exit tube. Just place it slightly above the surface of cold water. This tends to produce mists though, which is why SO3 is usually condensed neat or dissolved into concentrated sulfuric acid, then diluted with water. Were you using a rubber stopper? I sure hope not, because SO3 attacks everything and anything organic. By smell of sulfur, do you mean hydrogen sulfide or sulfur dioxide? I would suspect that either is coming from the stopper being destroyed if you used one. You should be using all glass (preferably fused quartz/vycor) apparatus. Concentrated sulfuric acid is an acceptable joint grease for this operation.
-

How does energy become electricity in electromagnetic induction?
UC replied to cameron marical's topic in Quantum Theory
Metal behaves like a "sea of electrons" some of the valence electrons on every atom in a piece of metal are more or less free to move about the stationary ions, which are positive charges. The net effect of this without external stimulus is that the electrons don't go anywhere. When you have a closed loop of conductor and you induce a current, you push the electrons in a definite direction. This is sort of like making water swirl around in a loop. As electrons drift one direction, those next to them drift into their original spot. It's all simultaneous. The positive and negative ends of batteries refer to which direction the electrons move relative to it, either in or out. With the crude analogy above, a battery is sort of like a pump. It can move the water around the loop in one direction. The fountain pump I use to chill my condenser, for example, has a listing of capabilities on it. The pumping rate gets lower the higher up the destination is from where the bottom of the pump sits. The same is true with voltage difference and current from a battery. Adding resistances in a circuit is like making the pump work uphill. It has a lowered rate of pumping (current). -
You list your location as england, thus I assume that your first language is english. Please take the time out of your busy day to type in it. Thank you.
-
No, it was ANFO that was used in the Oklahoma city bombing. By itself, ammonium nitrate is a horrible explosive. Even with fuel oil added to improve the oxygen balance, it still needs a very large initiator charge. ANFO is not something you'd want to use on the battlefield, but it is good for blasting because it's cheap and effective. Good explosives by comparison are glyceryl trinitrate, trinitrotoluene, pentaerythritol tetranitrate, etc. The newspaper is generally not a reliable source, especially for anything science related.
-
Deeeelicious misonformation. Ammonium nitrate is a crappy explosive. It is extremely hard to detonate pure, and even mixtures like ANFO need a large initiating charge. Some fertilizers come with coatings on the prills, though this is usually to aid wetting (as in sulfur, which normally repels water) or keep the pellets intact during transport. Waxy coatings are nothing more than a fuel if you were to try to detonate the stuff and would probably make it work slightly better. Also, ammonium nitrate prills intended for ANFO blasting (still used for mining operations) are not as compact as fertilizer prills (as dense as possible for cheaper shipping). They are slightly porous, which allows them to collapse as the detonation front propagates through the mixture. Fertilizer prills are harder to detonate because they are solid. When heated to the point of decomposition (and especially above that, as would be reached in a bunsen flame), it is much more sensitive to detonation. Using careful temperature control, N2O production is still done industrially by the same process. The sideproducts NO and NO2 are then thoroughly scrubbed from the mixture. I suspect that these would be formed in much higher proportions the hotter the reaction was. Wikipedia lists phosphates as catalysts for the formation of purer N2O at lower temperatures.
-
1) get some eggs. a lot of eggs. eat them. 2) get some hosing and vaseline. Hopefully you don't need instructions, on what to do with this, but lead your "exit gas stream" into cold water saturated with [ce] SO2 [/ce]. Sulfur will precipitate due to the following water-catalyzed reaction: [ce] 2H2S + SO2 -> 3S + 2H2O [/ce] Here is a nice video not involving flatus: Okay, so I'm obviously joking. The H2S content of farts is negligible. However, check out this: http://wiki.answers.com/Q/How_do_you_make_sulfur_at_home_what_stuff_do_you_need By far, the easiest way to get sulfur is to buy some. If you can get high quality anywhere, I suggest you do so, because trying to purify fertilizer grade by repeated recrystallization is a pain in the arse and very smelly since you need hot xylene or toluene for solvent, as well as a very fine metal screen as a filter. I've done it and seriously don't recommend it.
-
http://www.freepatentsonline.com/6706980.html According to this patent (and a few others out there), the problem is that gallium and indium oxide (even in very small amounts) massively lower the surface tension of the molten metal and allow it to adhere to other surfaces. The same is obviously not true of, or less true of mercury. I did notice this with gallium when melting it down. It came to me moderately oxidized and a bit grungy, but after cleaning, it stuck to platic a lot less when melted. I used a brand new pasteur pipette to break up the cleaned 20g of metal I had into some smaller pellets and it stuck, but not really badly to the glass.
-
The thing about permanganate is that is cleaves double bonds and completely oxidizes any functional groups that it can. You have the wrong reaction product there. As was already said, 3-hydroxypropanoic acid is the correct name. You could call this a beta-hydroxy acid, the simplest possible one, in fact.
-
I am familiar with schlenk lines, but with nitrogen instead of chlorine. Do you use a bubbler, and if so, what of? (mercury isn't chlorine-proof ) So, we have a dry, oxygen-free, nitrogen-free atmosphere of chlorine over 3-hydroxyglutaronitrile. What reaction (mechanism) takes place? (if you have a ref on hand, I'd be happy to read it over) As for safety, I more meant that the lab in question may never have a need for a lecture bottle of chlorine again, and if it ends up in storage for years, corrosion can be an issue. Granted, under ideal conditions, this would not be an issue, but who knows what to expect. As for hydrolysis of the cyano groups, were this occuring, the product would probably not be stable, especially in acidic, oxidizing conditions. See the orgsyn link in an earlier post, which claims total decomposition in a few hours in solution for acetone dicarboxylic acid. I suspect that 3-hydroxypentanedioic acid would not be much better off, especially if there is oxidant around. Personally, I would try the DMP as the next logical step. If that fails entirely, then maybe try something drastic.
-
If you have some decent glassware around, you should be able to construct a chlorine generator. You won't have high pressure available, but it's significantly less wait time, hazard, and cost than ordering a lecture bottle. Ideally, you have a pressure equalizing addition funnel charged with hydrochloric acid dripping into a 2-neck flask charged with trichloroisocyanuric acid (swimming pool chemical), calcium hypochlorite, N-chlorosuccinimide, KMnO4, MnO2, etc. The gas is lead through a CaCl2 drying tube before being used. A good writeup can be found here: http://www.sciencemadness.org/talk/viewthread.php?tid=9713
-
One breathful won't replace all the air in your lungs, it won't react with the oxygen at ordinary temperatures without catalysis, and it doesn't have an affinity for hemoglobin greater than oxygen like carbon monoxide does. I'd say that it isn't a great idea, but probably not going to kill you.
-
Also, see here for some information on the priority system: http://en.wikipedia.org/wiki/Cahn%E2%80%93Ingold%E2%80%93Prelog_priority_rules
-
Strong acids are still ionized. They protonate themselves in an equilibrium, For example, you'll fine the species [ce] H3SO4^+ [/ce] and it's counterion [ce] HSO4^- [/ce]. In fact, the number of ions is ten orders of magnitude higher than in high purity water (which is poorly conductive) http://en.wikipedia.org/wiki/Sulfuric_acid <- read the polarity and conductivity section. Cations tend to get reduced at the cathode, while anions tend to be oxidized at the anode. Some anions are incapable of further oxidation and many cations cannot be reduced effectively in aqueous solution because [ce] H^+ [/ce] as hydronium is easier to reduce than say, sodium. (also, the whole reacts with water thing )
-
What I didn't include in the above reaction diagrams is the movement of some hydrogens around, which occurs via interactions with the solvent, water. You could purify alpha-dextrose and it would last forever if you kept it dry in a bottle on a shelf, but a few hours in water and it'd be back to the mixture of forms. If you want to be more specific, the structure formed there is called a hemiacetal. Google can find you some in-depth reaction mechanisms if you ask it nicely
-

Synthesizing Elemental Iodine from Iodine Tincture
UC replied to hydraliskdragon's topic in Chemistry
1) Wear gloves, kid. 2) Try generating chlorine via a method other than removing the stopper and splashing in some HCl as I'm sure plenty escaped that way. A pressure-equalizing addition funnel in a ground glass filtering flask with some FEP or PFA tubing leading into the solution would be ideal. -
http://en.wikipedia.org/wiki/Nuclear_force Classical mechanics are not applicable on an atomic level. You'll be wanting quantum mechanics for that.
-
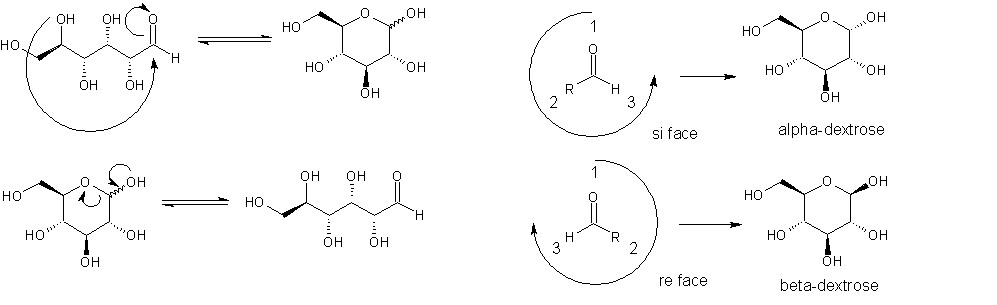
I'm not sure if you have enough chemistry background to know what I'm talking about when I discuss enantiomers and show electron pushing diagrams, but hopefully you do. Some of the information needed for enantiomers can be found on this wikipedia page and also in the linked thread: http://en.wikipedia.org/wiki/Cahn_Ingold_Prelog_priority_rules http://www.scienceforums.net/forum/showthread.php?p=493990#post493990 I have attached a simplified diagram of the conversion from ring to chain form of glucose. On the right side, I have replaced the carbohydrate tail of the aldehyde group by a simple R for clarity. What you need to understand about the aldehyde group is that it is planar. Therefore, the two sides look different and using the rules on that wiki page can be named as the re and si faces (pronounced "ray" and "psi") When the hydroxyl group attacks, it doesn't discriminate which side of the aldehyde it approaches. The reaction product is tetrahedral. However, the products of the hydroxyl adding to the different sides have different stereochemistry, which leads to alpha and beta dextrose. Now, if the reaction doesn't discriminate which side it attacks, why isn't the ratio of alpha to beta 1:1? Because the reverse reaction occurs readily, and since the alpha form is higher energy (less stable), the reverse reaction will happen more often for it, after which it has a 50:50 chance of becoming either an alpha or a beta. Eventually, you reach an equilibrium corresponding to the observed ratio.
-
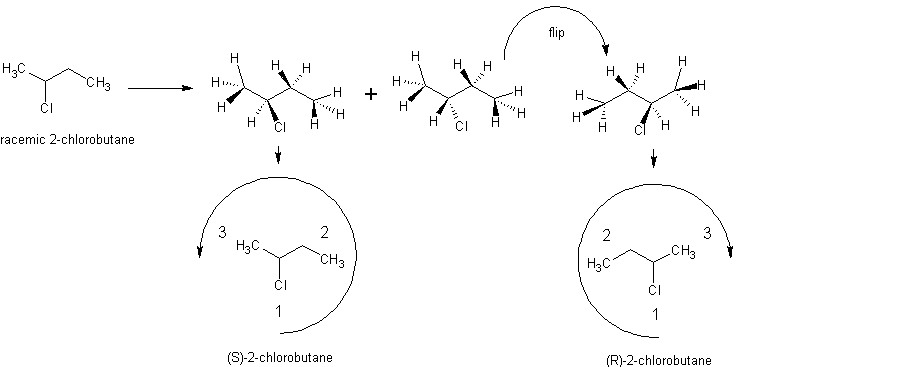
I like pretty pictures, how about you? On the left I have a typical drawing of 2-chlorobutane, which says nothing about it's chirality. I've labelled it as racemic, which means a 1:1 mix of the enantiomers. Many reactions produce racemates of enatiomeric products, but especially with pharmaceuticals (where only one enantiomer is biologically active), finding reactions that produce more of one than the other or only one of the two is important. Then, I've added the implicit hydrogens in, showing how all the carbons have a tetrahedral arrangement of bonds around them. The straight lines are in the plane of the screen while the solid lines project out toward you and the dashed lines project back. With this configuration visible, you can see the two non-superimposable forms of 2-chlorobutane. I then inverted everything (basically, looking at it from the other side) on the right enantiomer so that the hydrogen was pointing back into the page, as it already was on the left. Then I simplified the drawings, but maintained the relative positions of the the three substituents and labelled them according to priority. As you can see, motion around the stereocenter from highest to lowest priority defines the enantiomer. Of course, the lowest priority substituent doesn't have to be hydrogen. For 1-bromo-1-chloro-1-fluoroethane, the lowest priority substituent would be a methyl group, which would be treated the same way as hydrogen was above. For iodobromochlorofluoromethane, fluorine would be lowest priority, etc.
-
It's hard to choke and wheeze from water vapor ;0) "would sodium hydroxide remain dissolved in the water, even following evaporation?" No, but that means that a mist of water vapor is generated that was not vapor at some point. This is even more dramatic with the aluminum added than when just dissolving solid NaOH, but happens in both cases.
-
Have you ever done this reaction, or even dissolved NaOH in water? Are you aware of the cloud of sodium hydroxide-laden mist that forms and how incredibly hot it can get? A fairly simple distilled water scrubber would probably clean it up just fine though. Magnesium reacts fairly vigorously with vinegar and a dilute solution of sodium carbonate would be effective for scrubbing any acetic acid vapors from it. Best case is to go with YT, but Gallium is expensive, even if it is reusable.
-
Probably because carbon is cheap as dirt and platinum is $38/gram I would try using something other than aluminum. No approach to making a battery with it is not problematic in some way.